the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The older the richer: significant increase in breeding bird diversity along an age gradient of different coppiced woods
Lorenzo Mentil
Corrado Battisti
Giuseppe Maria Carpaneto
Forest structural complexity could be a good predictor of overall species diversity. Since tree harvesting has a negative effect on forest structure, it is important to analyse the effects of this disturbance on sensitive groups, as forest birds. In this study, we aimed to shed light on this aspect by analysing a set of univariate metrics in bird communities breeding in three coppiced forest habitats (coppiced of chestnut, coppiced of Turkey oak and high forest of beech) along a gradient in age classes. We hypothesised that, with increasing forest age, (i) breeding bird communities will progressively increase in diversity and, (ii) due to higher habitat heterogeneity due to coppicing, a higher species turnover in the first age classes could appear. In each forest habitat, all the metrics significantly increased, from recently coppiced to more mature forests, due to progressively higher availability of resources and niches along the gradient. When comparing paired forest habitats, abundance and richness were significantly different only in the two oldest age classes, highlighting that responses to different tree composition were more marked in the mature phase. In all forest habitats, species turnover (βw diversity) decreased progressively along the age gradient and was highest in the youngest age classes where many vegetation layers were present. Due to different coppice management practices, growth regime and consequent habitat heterogeneity, chestnuts showed a different pattern when compared to other forest habitats, with an increase in species turnover (βw diversity) at intermediate level. With increasing age of the forests, all the diversity metrics increased and species turnover decreased, highlighting the role of older forests as strategic habitats for highly structured bird communities.
- Article
(480 KB) - Full-text XML
-
Supplement
(174 KB) - BibTeX
- EndNote
Forests are universally recognised as a mosaic of complex and multifunctional ecosystems with intrinsic value. Therefore, forest management cannot be based only on the principles of the market economy but also on those of biodiversity conservation and ecosystem services (Lindenmayer et al., 2000; Siitonen, 2001; Pardini et al., 2005, 2010).
The structural complexity of a forest seems to be a good predictor of overall species diversity (Annand and Thompson, 1997; Dìaz et al., 2005). Accordingly, analysis of forest structures is known as a reliable criterion with which to assess the conservation value of forest stages (Doyon et al., 2005). Moreover, the structural changes of the forests have implications on their ecological functions, by alteration of the microclimate, the production of food resources and the capacity to provide shelter or nesting sites to animals (Wiens, 1989; Chapin III et al., 2000).
Forest birds, for their easy detectability and high sensitivity to human-induced environmental stress, are an important group for analysing the effects of forest ecosystem transformations (Blondel, 1975, 1981a; Wiens, 1989; Noss, 1990; Villard, 1998). Moreover, these animals depend very heavily on vegetation structure and their populations can be deeply affected by forest cutting (Ferry and Frochot, 1974; Canterbury et al., 2000; Jobes et al., 2004), one of the major anthropogenic threats recognised by the IUCN (International Union for Conservation of Nature, code 5.3 “Logging and wood harvesting”; review in Brawn et al., 2001; Battisti et al., 2016).
Tree harvesting has a negative effect on forest bird populations at different levels. Its intensity depends on spatial and soil features, as well as on the forest type (Donovan et al., 1997; Villard et al., 1999; Austen et al., 2001). Therefore, it is important to analyse the ecological effects of forest cuts, especially when these occur with high frequency and intensity as in the case of coppicing. Nevertheless, in southern Europe studies in this direction are few and rarely analyse the structural evolution of the differently characterised coppiced woods along age classes (Ciancio et al., 2006; Gil-Tena et al., 2007; Nascimbene et al., 2007; Torras and Saura, 2008; Spinelli et al., 2010).
The aim of this paper is to compare the structure of the bird communities in three different types of managed forests (Turkey oak coppicing, chestnut coppicing and beech high forest) belonging to different age classes in a regional nature reserve. Since these habitat types increase their structural complexity, moving from recently coppiced to high forest (and since structural complexity is a strong driving forces of resource and niche availability; Wiens, 1989), we hypothesised that breeding bird communities will progressively increase their univariate metrics of diversity, such as species abundance, richness and diversity. Moreover, since immediately after cutting, forest habitats become more heterogeneous, with the presence of open habitats and shrub vegetation, we hypothesised a higher species turnover (calculated with a β diversity metric) in the first age classes when compared to the more mature ones.
2.1 Study area
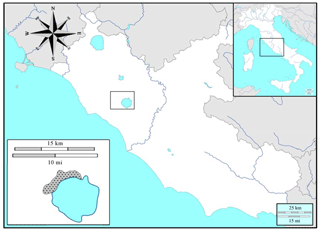
The study was carried out inside the Bracciano–Martignano Regional Park (provinces of Rome and Viterbo, central Italy, Lazio; Fig. 1), in the northern sector of the Sabatini Mountains (size: 3000 ha, geographic coordinates: N, E). This area include volcanic hills, with many pyroclastic cinder cones (e.g. M. Calvi, M. Termine, M. Rocca Romana) overlooking the caldera depressions, the largest of which harbours the lakes of Bracciano and Martignano. The altitude ranges between 164 m (Lake Bracciano) and 612 m a.s.l. (top of Mount Rocca Romana). The vegetation is dominated by broadleaf deciduous woodland, from oak to beech stands, with anthropogenic chestnut orchards (Sollevanti, 1983).
2.2 Subdivision of the forest habitat types and age classes
We studied three forest habitats: (1) coppiced woodlands of chestnut (Castanea sativa Miller) (total size area: 405 hectares), (2) coppiced woodlands of Turkey oak (Quercus cerris L.) and associated broadleaf deciduous trees (835 hectares) and (3) high forest of beech (Fagus sylvatica L.) (900 hectares), all managed by coppice management practices.
Forest habitats differ in structural traits due to different practices of coppice management: in particular, in chestnuts and Turkey oak woods traditional practices of coppicing were carried out with a specific time frequency for cutting (about 15–18 years). In contrast, beech forest were managed as high forest and practices are completely different (Ciancio et al., 2006). In southern Europe, high forest is a form of government characterised by a turn of about 90 years, followed by the birth of seedlings (details in Table 1).
Table 1Structural traits of forest habitats in the study area (see Methods for details). DBH is diameter at breast height.

For each forest habitat, we selected four age classes following the dynamic steps of their structure and local history of coppicing (Pregitzer and Euskirchen, 2004; Scarfò, 2012): the first three age classes belong to different succeeding steps of cutting, while the fourth step (an unmanaged wood) has been considered a control (i.e. woods with the oldest age when compared to coppicing turn; Scarfò, 2012; Table 1).
2.3 Protocol
Breeding bird communities was studied in the 2014 spring season (from March to May) with the point count method (Bibby et al., 2000). This method is particularly suitable for studies in patchy landscapes and in the spring period when species show territorial behaviour, so they can be easily detected (Blondel, 1981b; Sutherland, 2006). We used a 4 (age classes) × 3 (forest habitats) factorial design.
In each age class of each forest habitats, we located six point counts. Sampling points were randomly selected using a random number generator: http://stattrek.com/statistics/random-number-generator.aspx, last access: April 2014) and subsequently identifying the geographical coordinates using the free software Google Earth and a GPS Garmin E-trex on the field. We randomly located point counts (n=72). To evaluate the efficiency of our sampling, we built 12 data matrices, one for each site, corresponding to each species and treatment and then conducted a rarefaction analysis by building rarefaction curves with 95 % confidence intervals calculated after 9999 bootstraps (PAST 3.0 statistical software). Curves for age classes were grouped for each forest habitat. We observed rapid cumulating patterns in all treatments (outputs in Supplement Sect. S1): therefore we assumed that our sampling effort was representative of breeding bird assemblages, at least for common species.
Within each point count we recorded any individual bird seen and/or heard (song, call, drumming, alarm display), during a standardised fixed time (5 min), within a radius of 50 m. The use of this sampling distance was considered reliable for reducing the bias of detectability between mature and recently cut woodlands, where the probability of contacting birds is much broader and less sound absorbing because of dense vegetation (Sutherland, 2006).
In each point count we carried out two sampling sessions (session I: March, from 7 to 25; II: from 1 to 11 May 2014; total time effort: 720 min). We recorded birds in the morning from dawn to approximately 10:00. The distance among point counts remained at least 250 m to avoid counting the same individuals several times (pseudoreplication bias; see Rossi de Gasperis et al., 2016). For each species, in each point count, the highest value recorded in the two sampling sessions was considered valid (Blondel, 1975; Malavasi et al., 2009).
We used Nikon Monarch binoculars 12×42 DCF (roof prism with centre focus) and recorded calls using an iPhone 4s with a mini-microphone and the free software iTalk.
2.4 Data analyses
For each age class of each forest habitat, we obtained the species-specific abundance (number of individuals, n) and their relative frequency (Fr = n∕N, where N is the total number of individuals sampled). At the community level, we calculated the following univariate metrics of diversity: mean total abundance (Nmean), species richness (S), mean species richness (Smean) and Shannon–Wiener diversity index (as ; Shannon and Weaver, 1963; review in Magurran, 2004; Magurran and McGill, 2011).
Analysis has also been carried out at forest guild level (sensu stricto Verner, 1984), i.e. separately considering the species linked even partially with forest habitats (see Moore and Hooper, 1975; Cieslak, 1985; Opdam et al., 1985; Møller, 1987; Hinsley et al., 1995; Bellamy et al., 1996; Ukmar et al., 2007; list in Supplement Sect. S2) and calculating the percentage of their frequency in total for the whole assemblage (% FrFor).
To assess the level of intra-turnover of species inside the bird communities, we calculated the βw diversity index (Whittaker, 1972) as follows:
where γ is the total number of species recorded in the community (i.e. the γ diversity, here corresponding to the S value) and α is the averaged species richness (i.e. the α diversity, here corresponding to the Smean value).
To test if there are significant differences among mean values among multiple paired samples (i.e. samples with the same number of replicates, n=6), we carried out the non-parametric Friedman test. To test the differences between paired mean values, we carried out the non-parametric Wilcoxon test (Dytham, 2011). Alfa was set at 0.05. We used the free software PAST (Hammer et al., 2001).
In total, we recorded 868 individuals belonging to 30 species of breeding birds (checklist and data on abundances and on their frequencies at species level are reported in the Supplement Sect. S3).
Mean abundance and mean richness resulted in significant differences among the four age classes for all the forest habitats (Friedman test; Table 2), with highest values in class 4 at all sites. Analogously, Shannon–Wiener index also increased progressively from the youngest (1) to oldest (4) age classes.
Table 2Structural parameters of the bird community in the different forest habitats (Ch is chestnut; Oa is Turkey oak; Be is beech woods). S is species richness; S mean is mean species richness (and standard deviation, SD); N is total abundance; N mean is mean abundance (and SD); % FrFor is proportion (in percentage) of forest species frequency; H′ is Shannon–Wiener diversity index; βw is intra-habitat β diversity. Coefficient (χ2) of Friedman test and p values are reported. Significant values are in bold.
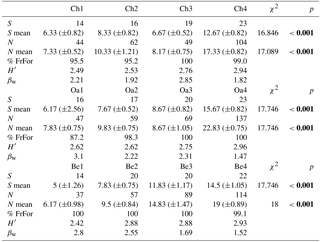
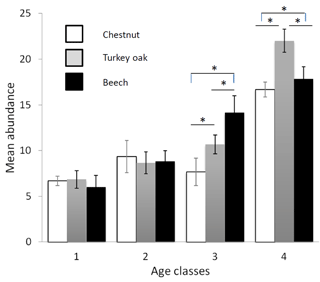
Figure 2Mean abundance of forest bird species (see Table 2) in the different forest habitats (age class and tree composition). Chestnut in white, Turkey oak in grey and beech in black. Error bars represent the standard deviation.
Bird communities were mainly composed of forest species, both qualitatively and quantitatively, with their frequency ranging between 87.2 % and 100 % of the total number of communities (Table 2). Considering only these species, we observed a significant difference among age classes in each forest habitat, both for mean abundance (chestnut: χ2=15.161, p=0.002; oak: χ2=17.746, p<0.001; beech: χ2=17.746, p<0.001, Fig. 2) and mean richness (chestnut: χ2=15.362, p=0.002; oak: χ2=17.431, p<0.001; beech: χ2=17.746, p<0.001, Fig. 3; Friedman test).
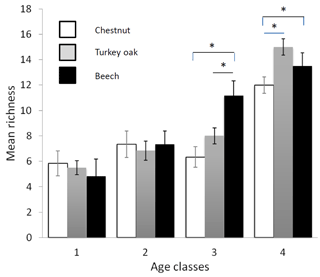
Figure 3Mean richness of forest bird species (see Table 2) in the different forest habitats (age class and tree composition). Chestnut in white, Turkey oak in grey and beech in black. Error bars represent the standard deviation.
Comparing mean abundance and mean richness between paired forest habitats for each age class, we observed significant differences (p<0.05) or p values tending to significance (p<0.09, Wilcoxon test) only at the higher age classes (3 and 4; Table 3). Comparing mean abundance and mean richness between contiguous age classes for different forest habitats, we observed significant differences at all sites (p<0.05, Wilcoxon test; Table 4).
Table 3Comparison of mean abundance and mean richness between paired forest habitats (Ch is chestnut; Oa is Turkey oak; Be is beech woods) for each age class (from 1 to 4). Z and p values (Wilcoxon test) are reported. Significant values are in bold.
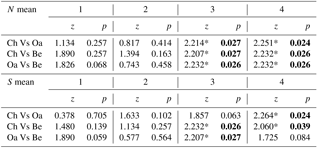
* p<0.01
Table 4Comparisons between mean abundance and mean richness between contiguous age classes (from 1 to 4) for different forest habitats. Z and p values (Wilcoxon test) are reported. Significant values are in bold.
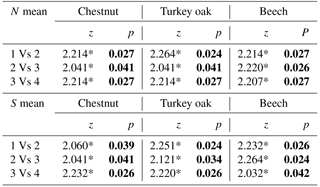
* p<0.01
Regarding intra-habitat βw turnover at wood patch level, the younger age classes of coppice (1 and 2) showed the highest values (i.e. the younger the age class, the more locally heterogeneous the habitat) compared to the oldest stages. We observed differences in pattern among forest habitats: indeed, in contrast to Turkey oak woods and beech forests (where βw species turnover was the highest in the age class 1), in chestnuts we showed an increase in species turnover in the forest renewal stage (age class 2; Table 2).
We observed a progressive and significant increase in diversity of breeding birds along an age gradient of different coppiced forest types. These results corroborated the general model that bird diversity metrics increase with the structural diversity of vegetation rather than its floristic composition (Johnston and Odum, 1956; Shugart and James, 1973; Shugart et al., 1975; Fuller and Henderson, 1992; Holmes and Sherry, 2001; Renfrew et al., 2005), following an analogous increase in resources and niches available for different species (see the relationship between abundance resources and richness niches; e.g. Ferry and Frochot, 1974; Blondel, 1981a; Maurer, 1986; Wiens, 1989; Martensen et al., 2008; Moning and Müller, 2009; Martensen et al., 2012; Sánchez et al., 2012; Bergner et al., 2015).
When we compared forest habitats, controlling for age class, we observed that mean abundance and richness significantly differ only in the most mature classes, while no difference occurs between recently cut woods (age classes 1 and 2). In the youngest age classes, forest habitats show a simplified structure which appears suitable only for a restricted number of species, which are more ecologically linked to shrubs, edge and open habitats and not specialised toward different tree species. Quantitative differences between forest habitats become significant only when these habitats markedly change their structure (oldest age classes: 3 and 4); i.e. when the woods grow older, the structural differences increase among different habitats, making new and different resources and niches available and favouring a quantitative differentiation in abundance and richness of bird communities.
Specifically, at species level, some hole-nesting species increases in abundance as tree size progressively increases, for example tits (Paridae), woodpeckers (Picidae), Certhia brachydactyla and Sitta europaea. On the contrary, some edge species inhabiting Mediterranean scrublands or coppices show an opposite trend, decreasing in abundance or disappearing with the increased forest age and structural complexity (e.g. Sylvia melanocephala, S. cantillans, Chloris chloris, Serinus serinus). Two species represent particular cases: Fringilla coelebs occurs only in the first and fourth ages of a forest, in both cases when tree density is low; conversely, Erithacus rubecula appears favoured by tree density, so its highest abundance values have been found in the intermediate ages.
Coppice management practices induce an increase in environmental heterogeneity at landscape and patch scale (Turner, 1989; McGarigal and McComb, 1995), affecting forest bird communities (Camprodon and Brotons, 2006; Castro et al., 2009; Dickson et al., 1993; Paillet et al., 2010). In our study, the younger age classes of coppice (1 and 2) also showed the highest level of intra-habitat βw turnover at wood patch level: therefore, the younger the age class, the more heterogeneous the habitat (hosting open areas, shrubs, isolated trees), in contrast to the oldest stages, where only mature trees with a closed canopy are present (see Webb et al., 1977; Crawford et al., 1981; McComb et al., 1989). However, we observed a different pattern among forest habitats. Indeed, in contrast to Turkey oak woods and beech forests (where βw species turnover was the highest in the youngest age class 1), in chestnuts we showed an increase in species turnover in the forest renewal stage (age class 2) due to different coppice management practices (Spinelli et al., 2010). Indeed, the physical structure of commercial coppiced chestnuts is substantially different from that of other types of broadleaved coppiced woods (Fuller and Moreton, 1987): in these woods, during the renewal stage there is rapid growth of the shrub layer with large leaves, increasing habitat heterogeneity and favouring many species linked to these new shady conditions (e.g. Turdus merula, Erithacus rubecula, Troglodytes troglodytes), with a consequent increase in species turnover at patch scale and at community level.
The bird communities associated with forest habitats are subject to different effects of forestry, depending on their level of specialisation (Dickson et al., 1993). Our communities were mainly composed of forest species. Therefore, patterns observed for overall bird assemblages have also been confirmed for the guild of strictly forest species: their structure significantly changed in all forest types, both in terms of mean abundance and richness along age groups, with a gradually increasing trend toward the older age class.
Forest species responses to structural changes were also evident. Forest species can be divided into two groups, (i) forest species sensu lato, i.e. generalist species linked to forest, wooded mosaics and wood-edge habitats, and (ii) forest interior species, i.e. specialised species that nest only within the interior of the forest and tend to avoid edge habitats (Whitcomb et al., 1981; Villard, 1998). The generalist forest species, such as tits (Paridae), Regulus ignicapilla, Certhia brachydactyla, Troglodytes troglodytes and Columba palumbus, are already present in the initial forest age (1 and 2) with very low abundance values, but grow with increasing forest age and structural complexity. A different case is the forest interior (sensu stricto Villard, 1998); for example woodpeckers (Picidae) and Sitta europaea are completely absent in the initial forest ages, appearing only in the fourth forest age in the case of coppice (chestnut and Turkey oak) and also in the intermediate forest ages in the high forest (beech). This is because in the intermediate forest ages of beech, woods aged over 30–50 years and they have characteristics suitable for these species. These species are known as interior hole-nesting birds and are highly sensitive to tree structure, forest changes and related disturbances (e.g. McCollin, 1993; Mikusinski and Angelstam, 1997; Matthysen, 1998; Pasinelli, 2007).
Our study analysed communities almost entirely composed of forest species and found an inverse pattern to that reported by Fuller and Moreton (1987) and Fuller and Henderson (1992), in which total abundance and diversity were highest in the youngest coppiced woods. Independently of tree species composition, older age class forests showed forest bird communities characterised by the highest values of diversity metrics (abundance, richness and Shannon–Wiener diversity), which were highly sensitive and specialised for more complex vegetation structure.
To conclude, our work shows that in forest bird communities when increasing age of the forests (i) habitat heterogeneity (βw turnover) decreased, (ii) all the diversity metrics increased, and (iii) differences in mean abundance and richness between different forest habitats become significant. Moreover, chestnuts showed a peculiar trend due to its characteristic pattern in tree growth.
However, these patterns could be dependent on forest types, on geographic and climatic conditions and contexts (Mediterranean area), on peculiar species composition (also due to distributional factors of species at regional scale) and on the local history of coppicing. Moreover, although our stratified sampling design allowed consistent data patterns to be obtained on different treatments (multiple age classes for each forest habitat), it is probable that increasing the number of sampling points for each treatment could add information on some rare species not recorded in our analysis. Therefore, long-term studies at wider spatial scales will be desirable to corroborate our patterns, by providing support to select sensitive bird species in managed forest landscapes (see Villard and Jonnson, 2009).
Data available only on personal request to the authors.
The supplement related to this article is available online at: https://doi.org/10.5194/we-18-143-2018-supplement.
LM, CB and GMC designed the study, LM carried out the field sampling, and LM and CB performed the analyses. All the authors wrote and revised the manuscript.
The authors declare that they have no conflict of interest.
We thank Andrea Cerulli (Parco Naturale Regionale di
Bracciano–Martignano) and Fabio Scarfò (Riserva Naturale Regionale
Monterano), for their information on local forest management and for
facilitations in the study areas. Two anonymous reviewers and the
editor-in-chief (Daniel Montesinos) largely improved the first draft of the manuscript with useful suggestions
and comments. Alessandro Zocchi reviewed the
English style and language.
Edited by: Daniel Montesinos
Reviewed by: two anonymous referees
Annand, E. M. and Thompson, F. R.: Forest bird response to regeneration practices in central hardwood forests, J. Wildlife Manage., 61, 159–171, 1997.
Austen, M. J. W., Francis, C. M., Burke, D. M., and Bradstreet, M. S. W.: Landscape context and fragmentation effects on forest birds in southern Ontario, Condor, 103, 701–714, 2001.
Battisti, C., Poeta, G., and Fanelli, G.: An introduction to disturbance ecology: a road map for wildlife management and conservation, Springer, The Netherlands, 2016.
Bellamy, P. E., Hinsley, S. A., and Newton, I.: Factors influencing bird species numbers in small woods in south-east England, J. Appl. Ecol., 33, 249–262, 1996.
Bergner, A., Avcı, M., Eryiğit, H., Jansson, N., Niklasson, M., Westerberg, L., and Milberg, P.: Influences of forest type and habitat structure on bird assemblages of oak (Quercus spp.) and pine (Pinus spp.) stands in southwestern Turkey, Forest Ecol. Manag., 336, 137–147, 2015.
Bibby, C. J., Burgess, N. D., Hill, D. A., and Mustoe, S.: Bird Census Techniques, 2nd Edn., Academic Press, London, 2000.
Blondel, J.: L'analyse des peuplements d'oiseaux, élément d'un diagnostique écologique, La méthode des échantillonnages fréquentiels progressifs (E.F.P.), Rev. Ecol.-Terre Vie, 29, 533–589, 1975.
Blondel, J.: Practical and theoretical problems of bird censusing in a mosaic of Mediterranean habitats, in: Censos de Aves en el Mediterraneo, edited by: Purroy, F. J., Proceeding 7 Int. Conf. Bird Census, V Meet. European Ornithology Atlas Committee, Leon, 57–63, 1981a.
Blondel, J.: Structure et dynamique des peuplements d'oiseaux forestiers, in: Actualites d'ecologie forestière, edited by: Pesson, P., Gauthier-Villars, Paris, 367–387, 1981b.
Brawn, J. D., Robinson, S. K., and Thompson, F. R.: III: The role of disturbance in the ecology and conservation of birds, Annu. Rev. Ecol. Syst., 32, 251–276, 2001.
Camprodon, J. and Brotons, L.: Effects of undergrowth clearing on the bird communities of the Northwestern Mediterranean Coppice Holm oak forests, Forest Ecol. Manag., 221, 72–82, 2006.
Canterbury, G. E., Martin, T. E., Petit, D. R., Petit, L. J., and Bradford, D. F.: Bird Communities and Habitat as Ecological Indicators of Forest Condition in Regional Monitoring, Conserv. Biol., 14, 544–558, 2000.
Castro, J., Moreno-Rueda, G., and Hódar, J. A.: Experimental Test of Postfire Management in Pine Forests: Impact of Salvage Logging versus Partial Cutting and Non-intervention on Bird-Species Assemblages, Conserv. Biol., 24, 810–819, 2009.
Chapin III, F. S., Zavaleta, E. S., Eviner, V. T., Naylor, R. L., Vitousek, P. M., Reynolds, H. L., Hooper, D. U., Lavorel, S., Sala, O. E., Hobbie, S. E., Mack, M. C., and Diaz, S.: Consequences of changing biodiversity, Nature, 405, 234–242, 2000.
Ciancio, O., Corona, P., Lamonaca, A., Portoghesi, L., and Travaglini, D.: Conversion of clearcut beech coppices into high forests with continuous cover: A case study in central Italy, Forest Ecol. Manag., 224, 235–240, 2006.
Cieslak, M.: Influence of forest size and other factors on breeding bird species number, Ekol. Pol.-Pol. J. Ecol., 33, 103–121, 1985.
Crawford, H. S., Hooper, R. G., and Titterington, R. W.: Songbird population response to silvicultural practices in central Appalachian hardwoods, J. Wildlife Manage., 45, 680–692, 1981.
Dìaz, I., Armesto Reid, J. J., Sieving, K. E., and Willson, M. F.: Linking forest structure and composition: avian diversity in successional forests of Chiloe Island, Chile, Biol. Conserv., 123, 91–101, 2005.
Dickson, J. G., Thompson, F. R., Conner, R. N., and Franzreb, E.: Effects of silviculture on neotropical migratory birds in central and south-eastern oak pine forests, in: Status and management of neotropical migratory birds: 21–25 September 1992, Estes Park, Colorado, edited by: Finch, D. M. and Stangel, P. W., Gen. Tech. Rep. RM-229, Fort Collins, Colo.: Rocky Mountain Forest and Range Experiment Station, U.S. Dept. of Agriculture, Forest Service, 374–385, 1993.
Donovan, T. M., Jones, P. W., Annand, E. M., and Thompson, F. R.: Variation in local-scale edge effects on cowbird distribution and nest predation, Ecology, 78, 2064–2075, 1997.
Doyon, F., Gagnon, D., and Giroux, J. F.: Effects of strip and single-tree selection cutting on birds and their habitat in a southwestern Quebec northern hardwood forest, Forest Ecol. Manag., 209, 101–115, 2005.
Dytham, C.: Choosing and using statistics: a biologist's guide, John Wiley & Sons, 2011.
Ferry, C. and Frochot, B.: L'influence du traitement forestier sur les oiseaux, Ecologie forestière (P. Pesson), Gauthier-Villars, Paris, 309–326, 1974.
Fuller, R. J. and Henderson, A. C. B.: Distribution of breeding songbirds in Bradfield Woods, Suffolk, in relation to vegetation and coppice management, Bird Study, 39, 73–88, 1992.
Fuller, R. J. and Moreton, B. D.: Breeding bird populations of Kentish sweet chestnut (Castanea sativa) coppice in relation to age and structure of the coppice, J. Appl. Ecol., 24, 13–27, 1987.
Gil-Tena, A., Saura, S., and Brotons, L.: Effects of forest composition and structure on bird species richness in a Mediterranean context: implications for forest ecosystem management, Forest Ecol. Manag., 242, 470–476, 2007.
Hammer, Ø., Harper, D. A. T., and Ryan, P. D.: Past: paleontological statistics software package for education and data analysis, Palaeontol. Electron., 4, 1–31, 2001.
Hinsley, S. A., Bellamy, P. E., Newton, I., and Sparks, T. H.: Habitat and landscape factors influencing the presence of individual breeding bird species in woodland fragments, J. Avian Biol., 26, 94–104, 1995.
Holmes, R. T. and Sherry, T. W.: Thirty-year bird population trends in an unfragmented temperate deciduous forest: importance of habitat change, Auk, 118, 589–609, 2001.
Jobes, A. P., Nol, E., and Voigt, D. R.: Effects of Selection Cutting on Bird Communities in Contiguous Eastern Hardwood Forests, J. Wildlife Manage., 68, 51–60, 2004.
Johnston, D. W. and Odum, E. P.: Breeding bird populations in relation to plant succession on the Piedmont of Georgia, Ecology, 37, 50–62, 1956.
Lindenmayer, D. B., Margules, C. R., and Botkin, D. B.: Indicators of Biodiversity for Ecologically Sustainable Forest Management, Conserv. Biol., 14, 941–950, 2000.
Magurran, A.: Measuring biological diversity, Blackwell Publishing, Malden, 2004.
Magurran, A. and McGill, B. J.: Biological diversity. Frontiers in measurements and assessments, Oxford University Press, Oxford, New York, 2011.
Malavasi, R., Battisti, C., and Carpaneto, G. M.: Seasonal bird assemblages in a Mediterranean patchy wetland: corroborating the intermediate disturbance hypothesis, Pol. J. Ecol., 57, 171–179, 2009.
Martensen, A. C., Pimentel, R., and Metzger, J. P.: Relative effects of fragment size and connectivity on bird community in the Atlantic Rain Forest: implications for conservation, Biol. Conserv., 141, 2184–2192, 2008.
Martensen, A. C., Ribeiro, M. C., Banks-Leite, C., Prado, P. I., and Metzger, J. P.: Associations of forest cover, fragment area, and connectivity with Neotropical understory bird species richness and abundance, Conserv. Biol., 26, 1100–1111, 2012.
Matthysen, E.: The Nuthatches, T & A.D. Poyser, London, 1998.
Maurer, B. A.: Predicting Habitat Quality for Grassland Birds Using Density-Habitat Correlations, J. Wildlife Manag., 50, 556–566, 1986.
McCollin, D.: Avian distribution patterns in a fragmented wooded landscape (North Humberside, U.K.): the role of between-patch and within-patch structure, Global Ecol. Biogeogr., 3, 48–62, 1993.
McComb, W. C., Groetsch, P. L., Jacoby, G. E., and McPeek, G. A.: Response of forest birds to an improvement cut in Kentucky, Proc. SE Assoc. Fish and Wild. Agencies, 43, 313–325, 1989.
McGarigal, K. and McComb, W. C.: Relationships Between Landscape Structure and Breeding Birds in the Oregon Coast Range, Ecol. Monogr., 65, 235–260, 1995.
Mikusinski, G. and Angelstam, P.: European woodpeckers and anthropogenic habitat change – a review, Die Wogenwelte, 118, 277–283, 1997.
Møller, A. P.: Variation in badge size in male house sparrows Passer domesticus: evidence for status signalling, Anim. Behav., 35, 1637–1644, 1987.
Moning, C. and Müller, J.: Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests, Ecol. Indic., 9, 922–932, 2009.
Moore, N. W. and Hooper, M. D.: On the number of bird species in British woods, Biol. Conserv., 8, 239–250, 1975.
Nascimbene, J., Marini, L., and Nimis, P. L.: Influence of forest management on epiphytic lichens in a temperate beech forest of northern Italy, Forest Ecol. Manag., 247, 43–47, 2007.
Noss, R. F.: Indicators for monitoring biodiversity: A hierarchical approach, Conserv. Biol., 4, 355–364, 1990.
Opdam, P., Rijsdijk, G., and Hustings, F.: Bird communities in small woods in an agricultural landscape: effects of area and isolation, Biol. Conserv., 34, 333–352, 1985.
Paillet, Y., Bergès, L., Hjältén, J., Ódor, P., Avon, C., Bernhardt-Römermann, M., Bijlsma, R. J., De Bruyn, L., Fuhr, M., Grandin, U., Kanka, R., Lundin, L., Luque, S., Magura, T., Matesanz, S., Mészáros, I., Sebastià, M. T., Schmidt, W., Standovár, T., Tóthmérész, B., Uotila, A., Valladares, F., Vellak, K., and Virtanen, R.: Does biodiversity differ between managed and unmanaged forests? A meta-analysis on species richness in Europe, Conserv. Biol., 24, 101–112, 2010.
Pardini, R., Marquez de Souza, S., Braga-Neto, R., and Metzger, J. P.: The role of forest structure, fragments size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape, Biol. Conserv., 124, 253–266, 2005.
Pardini, R., Bueno, A. A., Gardner, T. A., Prado, P. I., and Metzger, J. P.: Beyond the Fragmentation Threshold Hypothesis: Regime Shifts in Biodiversity Across Fragmented Landscapes, PLoS ONE, 5, e13666, https://doi.org/10.1371/journal.pone.0013666, 2010.
Pasinelli, G.: Nest site selection in middle and great spotted woodpeckers Dendrocopos medius & D. major: implications for forest management and conservation, Biodivers. Conserv., 16, 1283–1298, 2007.
Pregitzer, K. S. and Euskirchen, E. S.: Carbon cycling and storage in world forests: biome patterns related to forest age, Glob. Change Biol., 10, 2052–2077, 2004.
Renfrew, R. B., Ribic, C. A., and Nack, J. L.: Edge avoidance by nesting grassland birds: a futile strategy in a fragmented landscape, Auk, 122, 618–636, 2005.
Rossi de Gasperis, S., Redolfi De Zan, L., Battisti, C., Reichegger, I., and Carpaneto, G. M.: Distribution and abundance of hole-nesting birds in Mediterranean forests: impact of past management patterns on habitat preference, Ornis Fennica, 93, 100–110, 2016.
Sánchez, S., Cuervo, J. J., and Moreno, E.: Vegetation structure in beech-fir forests: effects on the avian community, Rev. Ecol.-Terre Vie, 67, 213–222, 2012.
Scarfò, F.: Effetti della gestione forestale sulla comunità ornitica nel Parco Regionale di Bracciano e Martignano (Lazio), XIV Convegno Italiano di Ornitologia – Trieste. Riv. Ital. Ornit., 82, 117–119, 2012.
Shannon, C. E. and Weaver, W.: Mathematical theory of communication, University of Illinois Press, Urbana, 1963.
Shugart, H. H. and James, D.: Ecological succession of breeding bird populations in northwestern Arkansas, Auk, 90, 62–77, 1973.
Shugart, H. H., Anderson, S. H., and Strand, R. H.: Dominant patterns in bird populations of the eastern deciduous forest biome. The management of forest and range habitats for game birds Symp, Proc. U.S. Dep. Agric. Gen Tech Rep. WO-1. U.S. Dep. Agic For Serv Washington, DC, 90–95, 1975.
Siitonen, J.: Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example, Ecol. Bull., 49, 11–41, 2001.
Sollevanti, F.: Geologic, volcanologic, and tectonic setting of the Vico-Cimino area, Italy, J. Volcanol. Geoth. Res., 17, 203–217, 1983.
Spinelli, R., Magagnotti, N., and Nati, C.: Benchmarking the impact of traditional small-scale logging systems used in Mediterranean forestry, Forest Ecol. Manag., 260, 1997–2001, 2010.
Sutherland, W. J.: Ecological Census Techniques: A Handbook, Cambridge, UK, Cambridge Univ. Press, 2006.
Torras, O. and Saura, S.: Effects of silvicultural treatments on forest biodiversity indicators in the Mediterranean, Forest Ecol. Manag., 255, 3322–3330, 2008.
Turner, M. G.: Landscape Ecology: The Effect of Pattern on Process, Annu. Rev. Ecol. Syst., 20, 171–197, 1989.
Ukmar, E., Battisti, C., Luiselli, L., and Bologna, M. A.: The effects of fire on communities, guilds and species of breeding birds in burnt and control pinewoods in central Italy, Biodivers. Conserv., 16, 3287–3300, 2007.
Verner, J.: The guild concept applied to management of bird populations, Environ. Manage., 8, 1–14, 1984.
Villard, M. A.: On forest-interior species, edge avoidance, area sensitivity, and dogmas in avian conservation, Auk, 115, 801–805, 1998.
Villard, M.-A. and Jonsson, B. G.: Setting Conservation Targets for Managed Forest Landscapes, Cambridge University Press, Cambridge, 2009.
Villard, M. A., Trzcinski, M. K., and Merriam, G.: Fragmentation effects on forest birds: Relative influence of woodland cover and configuration on landscape occupancy, Conserv. Biol., 13, 774–783, 1999.
Webb, W. L., Behrend, D. F., and Saisorr, B.: Effect of logging on songbird populations in a northern hardwood forest, Wildlife Monogr., 55, 6–35, 1977.
Whitcomb, R. E, Lynch, J. E., Klimkiewicz, M. K., Robbins, C. S., Whitcomb, B. L., and Bystrak, D.: Effects of forest fragmentation on avifauna of the eastern deciduous forest, in: Forest island dynamics in man-dominated landscapes, edited by: Burgess, R. L. and Sharpe, D. M., Springer-Verlag, New York, 125–205, 1981.
Whittaker, R. H.: Evolution and measurement of species diversity, Taxon, 21, 213–251, 1972.
Wiens, J. A.: The ecology of bird communities. Vol. 2. Processes and variations, Cambridge studies in ecology, Cambridge University Press, Cambridge, UK, 1989.