the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Feeding strategies differentiate four detritivorous curimatids in the Amazon
Gabriel Borba
Sidineia Amadio
Joicyeny Oliveira
Geraldo Santos
Adalberto Val
Efrem Ferreira
Differences in food intake and morphological attributes may facilitate the coexistence of detritivorous fish. The present study investigated the possible differences in the feeding strategies of four species of curimatids that inhabit the floodplain of the central Amazon. For this, we determined the diet, daily food cycle, and whether characteristics of the intestine were related to the length of the fish and the amount of detritus consumed. The detritivory was confirmed, and we observed a difference in the foraging time between species. We found differences in the length and weight of the intestine and the relationship of these variables with the length of the fish and the amount of detritus consumed. Our study suggests that despite belonging to the same family and food group, curimatids have characteristics that allow them to consume the detritus in different ways.
- Article
(771 KB) - Full-text XML
-
Supplement
(408 KB) - BibTeX
- EndNote
One of the ecological questions still unanswered about aquatic environments concerns the factors that determine species richness in detritivorous communities (Moore et al., 2004). How is it possible for the detritus to sustain a high quantity and diversity of fish in neotropical environments?
In the Amazon basin, one of the most representative detritivore groups is from the Curimatidae family, accounting for approximately half of the total fish biomass in South American rivers (Bowen, 1983; Lowe-McConnell, 1999; Vari and Röpke, 2013). This family has approximately 70 species. The species of the genera Psectrogaster and Potamorhina are the most abundant and widely distributed (Albert and Reis, 2011; Vari and Röpke, 2013; Van der Sleen and Albert, 2018). However, coexistence mechanisms are still poorly understood for this group. The species, in general, share the same environment and co-occur throughout the year, regardless of the hydrological cycle (Batista et al., 1998; Correia et al., 2015; Röpke et al., 2016). Besides, studies of feeding ecology have not encountered significant differences in the diets of these species, which are invariably dominated by detritus (Pereira and Resende, 1998; Vaz et al., 1999; Aranguren, 2002; Giora and Fialho, 2003; Alvarenga et al., 2006; Mérona et al., 2008).
Detritus is a highly available food resource, not limited to seasonal and spatial factors, and due to its structural characteristics, it may have been in the environment for millennia (Moore et al., 2004). It is defined as partially decomposed organic matter from plant and animal tissues, in addition to microorganisms and minerals (Gneri and Angeluscu, 1951; Gerking, 1994; Moore et al., 2004; Santana et al., 2015; Farrel et al., 2018; Zimmer, 2019). However, the chemistry of water and the spatial and seasonal availability of sources that form detritus is what will define its final composition (Bowen, 1983; Goulding et al., 1988).
The composition of the detritus (origin, quantity, and quality) can affect the feeding rate, population density, and trophic niche of detritivores (Rossi et al., 2015). However, niche overlap appears to be common for this food group. The fact that many species consume detritus and occupy the same environment, with an apparent lack of competition, seems to be promoted precisely by the abundance of the total resource and its high availability (Gerking, 1994; Pianka, 2000; Sidlauskas, 2007). However, the coexistence of detritivores can also be favored by differences in the use of this resource. The possible variety of detritus composition allows detritivores to specialize in food aggregates with different combinations of substrates (Delariva and Agostinho, 2001; Oliveira and Isaac, 2013; Rossi et al. 2015; Bayley et al., 2018). In addition, differences in detritus consumption can also occur in ontogenetic development (Semaprochilodus spp., Winemiller and Jepsen, 2004; Sarotherodon mossambicus, Bowen, 1979), during the reproductive period (Curimatella lepidura, Alvarenga et al., 2006), associated with foraging space or seasonality (Loricarichthys platymetopon, Lopes et al., 2009; Hypostomus spp., Oliveira and Isaac, 2013; Prochilodus spp., Bowen, 1983), by competition (Hypostomus spp., Oliveira and Isaac, 2013), or due to other physiological and/or ecological demands (Loricariidae, Lujan et al., 2011).
Independent of these conditions, all detritivorous fish have adaptations in the digestive process to extract large amounts of nutrients, since the detritus provides less energy and protein than other types of food (Sazima and Caramaschi, 1989; Bowen et al., 1995; Yossa-Pérdomo and Araújo-Lima, 1996; Castro and Vari, 2004; German and Bitong, 2009; Faria and Benedito, 2011). To enable a high rate of absorption and assimilation of nutrients, the digestive tract of these species is characterized by an extremely long intestine when compared to other feeding categories (Zihler, 1982; Smith, 1989; Kramer and Bryant 1995a; Karachle and Stergiou, 2010; Becker et al., 2010; Griffen and Mosblack, 2011; German et al., 2015). This characteristic, although general, can vary from 3 to 10 times the body size among detritivorous species (Moraes et al., 1997; Giora and Fialho, 2003; Alvarenga et al., 2006; German, 2009; Silva, 2016).
The differences found in the digestive tract, the type of detritus consumed, and foraging location is what defines the feeding strategy of each species. And this set of factors is closely aligned with feeding habits and phylogeny (Hidalgo et al., 1999; Guisande et al., 2012). Comparisons of the feeding strategies of closely related and sympatric species are still lacking, as is the case with curimatids. These species could present similar morphological characteristics, and they may nevertheless respond to the environment in different ways. They may present subtle shifts in certain traits, in particular those related to metabolism and digestive morphology (Ward-Campbell et al., 2005; Hilton et al., 2008; Mérona et al., 2008; Wagner et al., 2009; Griffen and Mosblack, 2011; Porreca et al., 2017). Thus, we investigated variations in the feeding strategy, specifically the relationship between morphological attributes and intake of detritus, of four abundant curimatid species in an aquatic environment in the central Amazon basin. Therefore, our objectives are to (1) confirm that the species investigated are detritivorous, (2) analyze variations in the daily food cycle, and (3) correlate morphological structures with feeding. We hypothesize that these variations in feeding strategy (even if subtle) could differentiate the consumption of detritus by the four species and facilitate their occurrence in the same ecological niche in Amazonian freshwater ecosystems.
2.1 Fish sampling
The curimatid species selected for the present study are the most abundant and frequently found in the Catalão region, a seasonally flooded area on the opposite margin of the Negro River from the city of Manaus (– S and – W). The four species were Potamorhina altamazonica (Cope, 1878), Potamorhina latior (Spix and Agassiz, 1829), Psectrogaster amazonica (Eigenmann and Eigenmann, 1889), and Psectrogaster rutiloides (Kner, 1858). We obtained the specimens analyzed in the present study from the Catalão/INPA Project (INPA – CEUA Ethics Committee, protocol 051/2015; IBAMA collecting license no. 52392-2). This project was initiated in 1999 and involves the collection of standardized monthly samples of specimens using a set of gill nets (mesh sizes ranging from 30 to 120 mm), which are positioned in the water for 24 h, with the fish being removed every 6 h (6, 12, 18, and 24 h). The specimens were taken to the Laboratory of Fish Population Dynamics (LDPP) at INPA in Manaus for identification, biometrics, and biological analyses.
2.2 Diet and the daily feeding cycle
We determined the composition of the diets of the specimens collected between June 2010 and July 2011 by two methods: (i) the frequency of occurrence (the percentage of the number of stomachs containing food that included the item) and (ii) the relative volume, based on a visual estimate of the percentage of the volume of each stomach taken up by the item (Hyslop, 1980). These values were multiplied by the estimated repletion of each stomach (0 %, 10 %, 25 %, 50 %, 75 %, or 100 %) to correct for errors resulting from the analysis of stomachs with different degrees of repletion (see Goulding et al., 1988; Ferreira, 1993). The values obtained by the two methods described above (frequency of occurrence and relative volume) were used to calculate the alimentary index (IAi) proposed by Kawakami and Vazzoler (1980): IA, where IAi is the food index of item i, Fi the frequency of occurrence of item i, and Vi the relative volume of item i.
We used the data collected between January 2013 and June 2018 to determine the daily feeding cycle. The time at which the specimen was retrieved from the net (i.e., 06:00, 12:00, 18:00, or 24:00 UTC−4) was considered to be a sampling point representative of the food ingested by the fish during the preceding 6 h. In this case, the stomach contents of a specimen collected at 06:00 UTC−4 were assumed to represent the food ingested between 24:00 and 06:00 UTC−4, while those of the specimens collected at 12:00 UTC−4 were considered to represent the food ingested between 06:00 and 12:00 UTC−4, those collected at 18:00 UTC−4 represent food ingested between 12:00 and 18:00 UTC−4, and those collected at 24:00 UTC−4 represent food ingested between 18:00 and 24:00 UTC−4. To control for the effect of the weight of the fish on the weight of its digestive tract, we calculated the digestive somatic index (DSI), given by DSI = (weight of digestive tract of the specimen∕total weight of the specimen) ×100.
2.3 Characterization of the digestive tract and morphological attributes
Here, we analyzed fish specimens collected between January 2018 and October 2019. We first measured the total length (TL) and standard length (SL) of each specimen in millimeters and its total weight (TW) in grams. We then obtained the weight of the intestine (with contents) and the total length of the intestine. We calculated the digestive somatic index (DSI = [weight of digestive tract weight∕total weight of specimen] ×100) as a measure of food consumed by each species.
2.4 Data analyses
We applied four statistical procedures to assess the differences among species in regarding morphological attributes and detritus intake. All analyses were performed with “log:log10”-transformed data due to the allometric relationship between the variables (Ricker, 1973; Wagner et al., 2009; Zandonà et al., 2015). First, we applied analysis of variance to test for the differences of morphological attributes among species. A one-way analysis was applied to each attribute: TL, TW, intestine length (IL), and intestine weight (IW).
The second analytical approach tested the difference in the relationships between morphological attributes (intestine length and weight) and the standard body length (SL) among the four species. We applied an analysis of covariance (ANCOVA) model for IL and IW whose SL, species identity, and the interaction of SL and species identity were the predictor variables.
The third analysis focused on the inference of the possible relationship between the amount of food ingested (DSI) and the structures of the digestive tract across the four species. The DSI was modeled based on the length and weight of the intestine as well as the interaction among these two variables. We set species identity as a factor to test whether estimates differ between species, and the log10-transformed standard length was included as a co-variate to control the differences in individual body sizes.
Then in the four analytical steps, we run a regression model for each morphological attribute (TL, TW, IL, IW, DSI) against the standard body size ignoring species identity and retained the residuals. To visualize the difference among species, we conducted a canonical variate analysis (CVA) with those extracted residuals from each morphological regression using the Morpho package. We also calculated and included a confidence ellipse (0.95) for each species, and the envfit function from the vegan package was used to fit morphological attributes onto the CVA ordination. To confirm the significant difference between species identity, we used the first canonical axis (summarizing 89 % of the total variation) as a dependent variable against the species identity in an ANOVA and a posteriori Tukey's test. All statistical tests were run in the R platform (R Core Team, 2020).
3.1 Diet and daily feeding cycle
We analyzed the stomach contents of 488 specimens of P. latior, 37 of P. altamazonica, 794 of P. rutiloides, and 33 of P. amazonica. We identified a variety of items including detritus (degraded organic matter), fragments of plant material, chlorophyte and cyanophyte algae, testate amoebae, ostracods, snails, cladocerans, and copepods. Overall, the intake of detritus exceeded 99 % of the frequency of occurrence (Table S1 in the Supplement). There were also no macroscopic differences in the detritus ingested by the species (Fig. S1 in the Supplement).
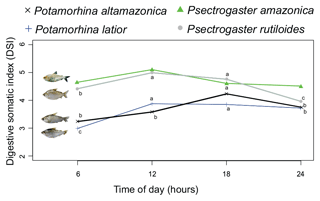
The daily feeding cycle was determined from the capture times of 127 specimens of P. latior, 114 P. altamazonica, 386 P. rutiloides, and 59 P. amazonica. The times indicated that all species were diurnal, feeding preferentially between 06:00 and 18:00 UTC−4, although with some intraspecific variation (Fig. 1). Potamorhina latior and P. rutiloides presented the highest digestive somatic index (DSI) between 12:00 and 18:00 UTC−4, whereas feeding in P. altamazonica peaked at 18:00 UTC−4. By contrast, the DSI of P. amazonica did not vary over the diurnal period. None of the analyzed specimens had an empty stomach or intestine, with DSI values invariably higher than 3 in the 06:00–18:00 UTC−4 period.
3.2 Characterization of the digestive tract and morphological attributes
The morphology of the digestive tract is similar in all four species (Figs. S2 and S3). The esophagus is a small tubular organ with a thin muscular wall, which thickens slightly where it joins the anterior portion of the stomach. The stomach is small, of fundic type, with a thin mucous layer covered by a thick muscular layer in the cardiac, fundic, and pyloric portions. The species of the genus Psectrogaster have a smaller, more rounded stomach (Fig. S3c), while in the Potamorhina species, this organ is larger and more elongated (Fig. S2c). In all four species, the intestine is extremely elongated and folded under the stomach, occupying most of the coelomic cavity (Figs. S2b and S3b). In P. latior and P. altamazonica, the diameter of the proximal portion of the intestine is enhanced, whereas in P. rutiloides and P. amazonica the intestine does not vary in width.
Morphological attributes varied among species. On average, P. altamazonica was the longest and heaviest intestine, and P. latior had the shortest intestine. Psectrogaster rutiloides had the lowest mean intestine weight, and Psectrogaster amazonica was the smallest species (Table 1).
Table 1Total length (TL), standard length (SL), total weight (TW), intestine weight (IW), and total intestine length (IL). Values are mean ± SEM. Comparison between species by analysis of variance with statistical differences presented by different lowercase letters.

Intestine length varied significantly among species (p<0.001), and P. altamazonica presented the longest intestine followed by P. amazonica, P. rutiloides, and P. latior (Table 1). Regression analysis (, p<0.001) showed that P. altamazonica (p=0.003) and P. latior (p=0.029) have significant variations in IL values. The relationship between intestine length and standard length was significant and positive for P. altamazonica (b=0.83, p<0.001), marginally significant for P. latior (b=0.46, p=0.06), and not significant for P. amazonica (b=0.83, p=0.08) and P. rutiloides (, p=0.59).
Intestine weight varied significantly among the analyzed species (p<0.001). Potamorhina altamazonica and P. rutiloides showed statistical differences between them (p<0.001) and between P. amazonica (p<0.001) and P. latior (p<0.001). Regression analysis (, p<0.001) showed that P. altamazonica (p=0.001) and P. latior (p<0.001) have significant variations in IW values. The relationship between intestine weight and standard length was significantly positive for P. altamazonica (b=1.60, p<0.001) and P. latior (b=0.65, p=0.03), negative for P. rutiloides (, p<0.001), and not significant for P. amazonica (, p=0.41) (Fig. 2b).
The relationship between the amount of food ingested (DSI) with the length (IL) and intestine weight (IW) was different among species (r2c=0.91). Potamorhina latior showed variation in DSI values (b=0.008 and p=0.002). The intestine weight showed a significant relationship with DSI (p=0.04) (Table 2).
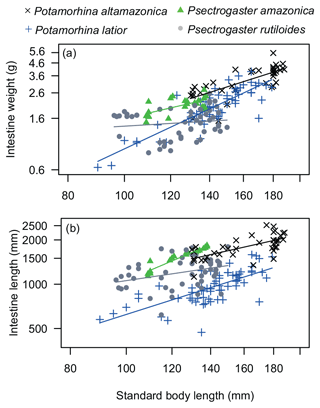
Figure 2ANCOVA regression plots (a) between SL (standard body length) and IW (intestine weight) and (b) between SL and IL (intestine length) of the four analyzed species of Curimatidae. Note that the variables were log10-transformed.
Table 2Results from the model between the amount of food ingested (DSI) and structures of the digestive tract from each species. The interaction between intestinal length (IL) and weight (IW) is included. Species identity was as a co-factor to test whether estimates differ between species, and the standard length (SL) was included as a random variable to control the differences in individual body sizes. All variables were log10-transformed. Bold values indicate significant variables in the model.

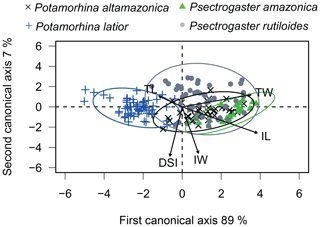
The canonical analysis (CAV) with the morphological attribute (TL, TW, IL, IW, and DSI) residuals showed 96 % of explained variation over the first two canonical axes. The first axis shows a significant difference between the four species (ANOVA, , p<0.01, Fig. 3). Overall, the four species are distinguished from one another, except P. altamazonica and P. rutiloides (Tukey's test; p>0.05). The ellipses of three species overlap, except P. latior, which shows an ellipsis in the opposite direction in the ordination space. Psectrogaster rutiloides presented individuals positively related to TW, IL, and IW. Potamorhina altamazonica and P. amazonica presented individuals positively related to a higher TW and IL, while P. latior was negatively associated with IL presenting lower values in the first CAV axis (Table S2).
We confirmed the occurrence of detritivory in all four species analyzed in the present study. The absence of macroscopic differences in the food consumed may support our hypothesis that variations in the feeding strategy differentiate the consumption of detritus and facilitate the occurrence of curimatids in the same ecological niche. The species constantly forage during the day, where there is an overlap of the peak foraging time, between 06:00 and 12:00 UTC−4, for the two species of Psectrogaster and P. latior. Similar patterns have been recorded for other curimatids and detritivores, which indicates that the overlapping of periods or forage sites may not inhibit their coexistence (Sazima and Caramaschi, 1989; Fugi et al., 1996; Oliveira and Isaac, 2013). Coexistence may be associated with the high availability of detritus (Moore et al., 2004; Zimmer, 2019): when food is unlimited, it is not a controlling factor in the abundance and distribution of fish, since there is no competition between species that feed on the same resource (Lowe-McConnell, 1999; Pianka, 2000). In this case, the space or time of foraging may be more limiting than the availability of food (Lowe-McConnell, 1999). Thus, P. altamazonica seeks alternative foraging times, reflecting a strategy to avoid competition for preferred foraging areas.
Although availability of detritus was not a limiting factor differences in the morphology of digestive attributes could be related to different feeding strategies (Karasov and Martinez Del Rio, 2007; German, 2011; Porreca et al., 2017). The digestive tract is formed by a small and muscular stomach and a very elongated intestine. This trait is common among curimatids and prochilodontids with similar feeding strategies (Al-Hussaini, 1949; Bowen, 1988; Yossa-Pérdomo and Araújo-Lima, 1996; Moraes et al., 1997; Silva, 2016). The small and muscular stomach, combined with the friction generated by the mineral component of the detritus, reduces the size of the particles, facilitating the action of digestive enzymes found in the intestine. The elongated intestine is folded extensively to fit inside the celomic cavity, resulting in a long intestinal passage time (Bowen, 1983; Smith, 1989; Bone and Moore, 2008; Griffen and Mosblack, 2011). This keeps the ingested detritus in contact with digestive enzymes for an extended period, ensuring the maximum possible extraction of nutrients (German, 2009; German and Bitong, 2009).
The similarity in the digestive tract is easily supported by the phylogenetic proximity and feeding strategies of the four species. These factors influence and determine the shape of the digestive tract (Hidalgo et al., 1999; Farrel et al., 2011; Guisande et al., 2012). However, our study found significant differences between the length and weight of the intestine as well as the amount of food consumed by the curimatids, which may be facilitating the coexistence of these fish. The length of the intestine varied significantly between the species studied. The longer the intestine, the greater the absorption surface, and the longer the time for enzymatic activity (Zihler, 1982; German, 2011). Therefore, the variation in the length of the intestine suggests differences in digestive physiology and in the time of assimilation of the detritus. Thus, the fact that P. latior has a shorter intestine reflects a strategy that consists of less time spent absorbing nutrients and/or consuming easily digestible items.
The relationship between gut length and the standard length was also contrasting between species. For P. altamazonica, the larger the individual, the longer the intestine; however for P. latior and P. amazonica, this relationship is not significant, and, for P. rutiloides, even negative, although not significant. Although the IL versus SL relationship is not clear to the curimatids in this study, it was recognized in other detritivores (Fugi et al., 2001, b=0.961; Angeluscu and Gneri, 1949, apud Kramer and Bryant, 1995b, b=1.06) Therefore, consuming comparable food items may not be the only factor that determines this relationship. For example, Kramer and Bryant (1995b) found that herbivorous species showed wide variation in the slope of the IL × SL ratio (b=1.36–2.11) and concluded that this is due to different ways of digesting the same food item. Thus, factors other than diet can influence this relationship and should be investigated such as the physiological and ecological processes (Kramer and Bryant, 1995b; Mérona et al., 2008; Zandonà et al. 2015).
Intestinal weight also varied between species, with P. rutiloides being the species with the lowest value. The amount of food present in the digestive tract during each feeding may or may not be related to the length of the intestine (Starck, 2003; Barboza et al., 2010; German, 2011). However, only P. altamazonica obtained a positive association with high values of weight and length of the intestine. For the other species, this was not observed. Thus, in addition to length, other characteristics of the intestine (weight, number of folds and microvilli, etc.) must be considered to determine the intestinal capacity to accommodate food. There was also no pattern for species in the IW versus SL ratio. Psectrogaster amazonica did not present a clear pattern; for P. altamazonica and P. latior, the larger the size of the fish, the heavier the intestine, indicating that larger individuals can consume and/or store more food. Psectrogaster rutiloides showed a negative relationship, indicating that larger individuals do not ingest/store more detritus. This negative relationship in P. rutiloides may also be related to the consumption of a different type of detritus or greater potential for the assimilation of nutrients in larger individuals. Farago (2018, unpublished data) showed that this species may be able to digest lipids up to 20 times more efficiently than other curimatids. In either case, P. rutiloides would have a strategy capable of extracting more energy from a smaller amount of detritus stored in the intestine.
The influence of the type and amount of food in the trophic niche of detritivores has been observed in other cases, and this seems to allow species in this group to specialize in discrete combinations of detritivorous compounds (Delariva and Agostinho, 2001; Constantini and Rossi, 2010; Oliveira and Isaac, 2013; Rossi et al., 2015; Santos et al., 2020). The species showed an association of their intestinal weight with the amount of food ingested (DSI). In curimatids, higher consumption of detritus tends to reflect in a heavier intestine. Potamorhina latior was the only species to have a significant and slightly positive relationship with the DSI, suggesting that it can compensate for its shorter intestine with higher consumption of detritus. This strategy would increase the amount of food items that pass through the digestive tract, allowing greater assimilation of nutrients (German, 2011).
Our results from the morphological attributes confirm the distinction between the curimatids. Even there is an overlapping between species, the intestine length (IL) was the main attribute that differentiated them. As this characteristic is directly related to digestive efficiency (Karasov and Martinez del Rio, 2007; Karachle and Stergiou, 2010; Griffen and Mosblack, 2011; German, 2011), it can be a relevant point to determine the different feeding strategies of species. The shorter intestine of P. latior suggests that the detritus consumed by this species needs less contact with digestive enzymes and, perhaps, less intestinal transit time (Smith, 1989; Karasov and Martinez del Rio, 2007; German, 2011). Despite the species of Curimatidae showing similar morphological adaptations to a diet restricted to detritus (Vari, 1989; Guisande et al., 2012; Melo et al., 2018), differences like this demonstrate that the evolutionary path to detritivory may not be the same for these fishes.
The data are available from the authors by personal request.
The supplement related to this article is available online at: https://doi.org/10.5194/we-20-133-2020-supplement.
TF, SA, GdS, AV, and EF designed the study. TF and JO collected the specimens in the field. TF and GB conducted the analyses. All the authors contributed, reviewed, and approved the final version of the manuscript.
The authors declare that they have no conflict of interest.
We thank the Brazilian National Institute for Research of the Amazon (INPA), the Institutional Research Project: Ecology and Conservation of Freshwater Fish (PPI/INPA), and the Fish Population Dynamics Laboratory for providing logistic and continued support over the year of this study. We thank, in particular, Cristhiana P. Röpke for all the assistance provided throughout the submission process. We also thank the local fishers and all the other people involved in this research in some way.
This research has been supported by (1) INCT Adapta: Adaptations of the Amazonian Aquatic Biota – CNPq/FAPEAM/CAPES (016/2014); (2) FAPEAM Universal Program (021/2011): Dinâmica trófica da assembleia de peixes e sua variação dentro de um ciclo hidrológico em uma área da Amazônia Central, Amazonas; and (3) Long-Term Ecological Research Program PELD-DIVA (015/2016): Fish diversity in response to different types of management in flooded areas of Central Amazonia: ecological and socio-economic aspects.
This paper was edited by Roland Brandl and reviewed by Roland Brandl and one anonymous referee.
Albert, J. S. and Reis, R. E.: Historical Biogeography of Neotropical Freshwater Fishes, University of California Press, London, 2011.
Al-Hussaini, A. H.: On the functional morphology of the alimentary tract of some fish in relation to differences in their feeding habits: anatomy and histology, Q. J. Microsc. Sci., 90, 109–139, 1949.
Alvarenga, E. R., Bazzoli, N., Santos, G. B., and Rizzo, E.: Reproductive biology and feeding of Curimatella lepidura (Eigenmann & Eigenmann) (Pisces, Curimatidae) in Juramento reservoir, Minas Gerais, Brazil, Rev. Bras. Zoo., 23, 314–322, 2006.
Angeluscu, V. and Gneri, F. S.: Adaptaciones del aparato digestivo al régimen alimenticio en algunos peces del Rio Uruquay y del Rio de La Plata, Revista del Instituto Nacional de Investigación de las Ciencias Naturales, 1, 161–272, 1949.
Aranguren, L. C. N.: Alimentação de Potamorhina latior (Spix, 1829) (Characiformes: Curimatidae) e Anodus elongatus (Agassiz, 1829) (Characiformes: Hemiodontidae) em lagos marginais do rio Acre-Amapá (AC) e Pirapora (AM), PhD thesis, Universidade Federal de São Carlos, Brazil, 154 pp., 2002.
Barboza, P. S., Bennet, A., Lignot, J. H., Mackie, R. I., McWhorter, T. J., Secor, S. M., Skovgaard, N., Sundset, M. A., and Wang, T.: Digestive Challenges for Vertebrate Animals: Microbial Diversity, Cardiorespiratory Coupling, and Dietary Specialization, Physiol. Biochem. Zool., 83, 764–774, 2010.
Batista, V. S., Inhamuns, A. J., Freitas, C. E. C. and Freire-Brasil, D.: Characterization of the fishery in river communities in the low-Solimões/high-Amazon region, Fisheries Manag. Ecol., 5, 419–435, 1998.
Bayley, P. B., Castello, L., Batista, V. S., and Fabré, N. N.: Response of Prochilodus nigricans to flood pulse variation in the central Amazon, R. Soc. Open Sci., 5, 172232, https://doi.org/10.1098/rsos.172232, 2018.
Becker, A. G., Gonçalves, J. F., Garcia, L. O., Behr, E. R., Graça, D. L., Filho, M. K., Martins, T., and Baldisseroto, B.: Morphometric parameters comparisons of the digestive tract of four teleosts with different feeding habits, Ciência Rural, 40, 862–866, 2010.
Bone, Q. and Moore, R. H.: Biology of fishes, 3rd. Edn., Taylor and Francis Group, New York, 2008.
Bowen, S. H.: A Nutritional Constraint in Detritivory by Fishes: The Stunted Population of Sarotherodon mossambicus in Lake Sibaya, South Africa, Ecol. Monogr., 49, 17–31, 1979.
Bowen, S. H.: Detritivory in neotropical fish communities, Environ. Biol. Fish., 9, 137–144, 1983.
Bowen, S. H.: Detritivory and Herbivory, in: Biologie et Ecologic des Poissons d'eau deuce africains, edited by: Lev-Cque, L., Bruton, M. N. and Ssentogo, G. W., Trav. et Dot., ORSTOM, 243–247, 1988.
Bowen, S. H., Lutz, E. V., and Ahlgren, M. O.: Dietary protein and energy as determinants of food quality: trophic strategies compared, Ecology, 76, 899–907, 1995.
Castro, R. M. C. and Vari, R. P.: Detritivorous of the South American fish family Prochilodontidae (Teleostei: Ostariophysi: Characiformes): A phylogenetic and revisionary study, Sm. C. Zool., 622, 1–200, 2004.
Constantini, M. L. and Rossi, L.: Species diversity and decomposition in laboratory aquatic systems: the role of species interactions, Freshwater Biol., 55, 2281–2295, 2010.
Correia, G. B., Siqueira-Souza, F. K., and Freitas, C. E. C: Intra- and inter-annual changes in the condition factors of three Curimatidae detritivorous from Amazonian floodplain lakes, Biota Neotrop., 15, 1–7, 2015.
Delariva, R. L. and Agostinho, A. A.: Relationship between morphology and diets of six neotropical loricariids, J. Fish Biol., 58, 832–847, 2001.
Farago, T. L. B: Utilização do detrito por espécies de peixes amazônicas: assimilação diferencial e partilha de recurso, PhD, thesis, Instituto Nacional de Pesquisas do Amazonas, Brazil, 139 pp., 2018.
Faria, A. C. A. and Benedito, E.: Quality and digestibility of food ingested by various trophic fish groups in the Upper Paraná River floodplain, Rev. Biol. Trop., 59, 85–101, 2011.
Farrel, A. P., Stevens, E. D., Cech, J. J., and Richards, J. G.: Encyclopedia of fish physiology: from genome to environment, Elsevier/Academic Press, Amsterdam, 2011.
Farrel, K. J., Rosemond, A. D., Kominoski, J. S., Bonjour, S. M., Rüegg, J., Koening, L. E., Baker, C. L., Trentman, M. T., Harms, T. K., and McDowell, W. H.: Variation in detrital resource stoichiometry signals differential carbon to nutrient limitation for stream consumers across biomes, Ecosystems, 21, 1676–1691, https://doi.org/10.1007/s10021-018-0247-z, 2018.
Ferreira, E. J. G.: Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil, Acta Amazon., 23, 1–89, 1993.
Fugi, R., Hahn, N. S., and Agostinho, A. A.: Feeding styles of five species of bottom-feeding fishes of the high Paraná River, Environ. Biol. Fish., 46, 297–307, 1996.
Fugi, R., Agostinho, A. A., and Hahn, N. S.: Trophic morphology of five benthic-feeding fish species of a tropical floodplain, Rev. Brasil. Biol., 61, 27–33, 2001.
Gerking, S. D.: Feeding Ecology of Fish, Academic Press, San Diego, 416 pp., 1994.
German, D. P.: Inside the guts of wood-eating catfishes: can they digest wood?, J. Comp. Physiol. B, 179, 1011–1023, 2009.
German, D. P.: Digestive Efficiency. In: Encyclopedia of Fish Physiology: From Genome to Environment, Farrell A.P., 1596–1607, 2011.
German, D. P. and Bittong, R. A.: Digestive enzyme activities and gastrointestinal fermentation in wood-eating catfishes, J. Comp. Physiol., 179, 1025–1042, 2009.
German, D. P., Sung, A., and Jhaveri, A. R.: More than one way to be an herbivore: convergent evolution of herbivory using different digestive strategies in prickleback fishes (Stichaeidae), Zoology, 118, 161–170, 2015.
Giora, J. and Fialho, C. B.: Biologia alimentar de Steindachnerina brevipinna (Characiformes, Curimatidae) do rio Ibicuí-Mirim, Rio Grande do Sul, Brasil, Iheringia Série Zoologica, 93, 277–281, 2003.
Gneri, F. S. and Angeluscu, V.: La nutrición de los peces iliófagos – En relación con el metabolismo general del ambiente acuático, Rev. Inst. Nac. Inv. Cs. Nat., 11, 1–38, 1951.
Goulding, M., Carvalho, M. L., and Ferreira, E. G.: Rio Negro, Rich life in poor water – Amazonian diversity and foodchain ecology as seen through fish communities, Academic publishing, The Netherlands, 1988.
Griffen, B. D. and Mosblack, H.: Predicting diet and consumption rate differences between and within species using gut ecomorphology, J. Anim. Ecol., 80, 854–863, 2011.
Guisande, C., Pelayo-Villamil, P., Vera, M., Manjarrés-Hernández, A., Carvalho, M. R., Vari, R. P., Jiménez, L. F., Fernández, C., Martínez, P., Prieto-Piraquive, E., Granado-Lorencio, C., and Duque, S. R.: Ecological Factors and Diversification among Neotropical Characiforms, Int. J. Ecol., 20, 1–12, 2012.
Hidalgo, M. C., Urea, E., and Sanz, A.: Comparative study of digestive enzymes in fish with different nutritional habits: Proteolytic and amylase activities, Aquaculture, 170, 267–283, 1999.
Hilton, Z., Wellenreuther, M., and Clements, K. D.: Physiology underpins habitat partitioning in a sympatric sister-species pair of intertidal fishes, Funct. Ecol., 22, 1108–1117, 2008.
Hyslop, E. J.: Stomach contents analysis – a review of methods and their application, J. Fish Biol., 17, 411–429, 1980.
Karachle, P. K. and Stergiou, K. I.: Intestine morphometrics of fishes: a compilation and analysis of bibliographic data, Acta Ichthyol. Piscat., 40, 45–54, 2010.
Karasov, W. H. and Martinez del Rio, C.: Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins, Princeton University Press, Princeton, 2007.
Kawakami, E. and Vazzoler, G.: Método gráfico e estimativa de índice alimentar aplicado no estudo de alimentação de peixes, Bol. Inst. Oceanogr., 29, 205–207, 1980.
Kramer, D. L. and Bryant, M. J.: Intestine length in the fishes of a tropical stream: 1. Ontogenetic allometry, Env. Biol. Fish, 42, 115–127, 1995a.
Kramer, D. L. and Bryant, M. J.: Intestine length in the fishes of a tropical stream: 2. Relationships to diet – the long and short of a convoluted issue, Env. Biol. Fish, 42, 129–141, 1995b.
Lopes, C. A., Benedito, E., and Martinelli, L. A.: Trophic position of bottom-feeding fish in the Upper Paraná River floodplain, Braz. J. Biol., 69, 573–581, 2009.
Lowe-McConnell, R. H.: Estudos ecológicos de comunidades de peixes tropicais, São Paulo, EDUSP, 1999.
Lujan, N. K., German, D. P., and Winemiller, K. O.: Do wood-grazing fishes partition their niche? Morphological and isotopic evidence for trophic segregation in Neotropical Loricariidae, Funct. Ecol., 25, 1327–1338, 2011.
Melo, B. F., Sidlauskas, B. L., Hoekzema, K., Vari, R. P., Dillman, C. B., and Oliveira, C.: Molecular phylogenetics of Neotropical detritivorous fishes of the Family Curimatidae (Teleostei: Characiformes), Mol. Phylogenet. Evol., 127, 800–812, 2018.
Mérona, B., Hugueny, B., Tejerina-Garro, F. L., and Gautheret, E.: Diet-morphology relationship in a fish assemblage from a medium-sized river of French Guiana: the effect of species taxonomic proximity, Aquat. Living Resour., 21, 171–184, 2008.
Moore, J. C., Berlow, E. L., Coleman, D. C., Ruiter, P. C., Dong, Q., Hastings, A., Johnson, N. C., McCann, P.J., Melville, K., Morin, P. J., Nadelhoffer, K., Rosemond, A. D., Post, D. M., Sabo, J. L., Scow, K. M., Vanni, M. J., and Wall, D. H.: Detritus, trophic dynamics and biodiversity, Ecol. Lett., 7, 584–600, 2004.
Moraes, M. F. P. G., Barbola, I. F., and Guedes, E. A. C.: Alimentação e relações morfológicas com o aparelho digestivo do “curimbatá”, Prochilodus lineatus (Valenciennes) (Osteichthyes, Prochilodontidae), de uma lagoa do Sul do Brasil, Rev. Bras. Zool., 14, 169–180, 1997.
Oliveira, J. C. S. and Isaac, V. J.: Diet breadth and niche overlap between Hypostomus plecostomus (Linnaeus, 1758) and Hypostomus emarginatus (Valenciennes, 1840) (Siluriformes) in the Coaracy Nunes hydroelectric reservoir, Ferreira Gomes, Amapá-Brazil, Biota Amazonia, 3, 116–125, 2013.
Pereira, R. A. C. and Resende, E. K.: Peixes detritívoros da planície inundável do rio Miranda, Pantanal, Mato Grosso do Sul, Brasil, EMBRAPA-CPAP, Corumbá, 1998.
Pianka, E. R.: Evolutionary ecology, San Francisco, Benjamin/Cummings, 2000.
Porreca, A. P., Hintz, W. D., Coulter, D. P., and Garvey, J. E.: Subtle physiological and morphological differences explain ecological success of sympatric congeners, Ecosphere, 8, 1–14, 2017.
R Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, available at: https://www.R-project.org/, last access: June 2020.
Ricker, W. E.: Linear Regressions in Fishery Research, J. Fish. Res. Board. Can., 30, 409–434, 1973.
Röpke, C. P., Amadio, S., Winemiller, K. O., and Zuanon, J.: Seasonal dynamics of the fish assemblage in a floodplain lake at the confluence of the Negro and Amazon Rivers, J. Fish Biol., 89, 194–212, 2016.
Rossi, L., Lascio, A., Carlino, P., Calizza, E., and Constantini, M. L.: Predator and detritivore niche width helps to explain biocomplexity of experimental detritus-based food webs in four aquatic and terrestrial ecosystems, Ecol. Complex., 23, 14–24, 2015.
Santana, A. R., Werth, M., and Benedito-Cecílio, E.: Use of food resource by detritivorous fish in floodplains: a synthesis, Acta Biol. Col., 20, 5–14, 2015.
Santos, N. C. L., Dias, R. M., Alves, D. C., Melo, B. A. R., Ganassin, J. M., Gomes, L. C., Severi, W., and Agostinho, A. A.: Trophic and limnological changes in highly fragmented rivers predict the decreasing abundance of detritivorous fish, Ecol. Indic., 110, 105933, https://doi.org/10.1016/j.ecolind.2019.105933, 2020.
Sazima, I. and Caramaschi, E. P.: Comportamento alimentar de duas espécies de Curimata, sintópicas no Pantanal de Mato Grosso (Osteichthyes, Characiformes), Rev. Bras. Biol., 49, 325–333, 1989.
Sidlauskas, B. L.: Testing for unequal rates of morphological diversification in the absence of a detailed phylogeny: a cause study from Characiform fishes, Evolution, 61, 299–316, 2007.
Silva, L. T.: Adaptações morfológicas do trato digestório do peixe neotropical Steindachnerina notonota (Characiformes, Curimatidae) ao hábito alimentar detritívoro, PhD, thesis, Universidade Federal do Rio Grande do Norte, Brazil, 116 pp., 2016.
Smith, L. S.: Digestive functions in teleost fishes, in: Fish nutrition, 2nd Edn., edited by: Halver J. E., Academic Press, London, 331–421, 1989.
Starck, J. M.: Shaping up: how vertebrates adjust their digestive system to changing environmental conditions, Anim. Biol., 53, 245–257, 2003.
Van der Sleen, P. and Albert, J. A.: Field guide to the fishes of the Amazon, Orinoco and Guianas, Princeton University Press, New Jersey, 2018.
Vari, R. P.: A phylogenetic study of the Neotropical Characiform Family Curimatidae (Pisces: Ostariophysi), Sm. C. Zool., 471, 1–80, 1989.
Vari, R. P. and Röpke, C. P.: Curimatidae, in: Peixes do Rio Madeira, edited by: Queiroz, L. J., Torrente-Vilara, G., Ohara, W. M., Pires, T. H. S., Zuanon, J., and Doria, C. R., 1st Edn., Dialeto, São Paulo, 112–139, 2013.
Vaz, M. M., Petrere Jr., M., Martinelli, L. A., and Mozeto, A. A.: The dietary regime of detritivorous fish from the River Jacaré Pepira, Brazil, Fisheries Manag. Ecol., 6, 121–132, 1999.
Wagner, C. E., McIntyre, P. B., Buels, K. S., Gilbert, D. M., and Michel, El.: Diet predicts intestine length in Lake Tanganyika's cichlid fishes, Funct. Ecol., 23, 1122–1131, 2009.
Ward-Campbell, B. M. S., Beamish, F. W. H., and Kongchaiya, C.: Morphological characteristics in relation to diet in five coexisting Thai fish species, J. Fish Biol., 67, 1266–1279, 2005.
Winemiller, K. O. and Jepsen, D.B.: Migratory Neotropical Fish Subsidize Food Webs of Oligotrophic Blackwater Rivers, J. Fish. Biol., 53, 267–296, 2004.
Yossa-Pérdomo, M. I. and Araújo-Lima, C. A. R. M.: The quality of the detritus used by amazonian fish during the low water season, in: Physiology of Tropical Fish Symposium Proceedings, International Congress on the Biology of Fishes Bethesda, MD: American Fisheries Society, Physiology Section, 82–83, 1996.
Zandonà, E., Auer, S. K., Kilham, S. S., and Reznick, D. N.: Contrasting Population and Diet Influences on Gut Length of an Omnivorous Tropical Fish, the Trinidadian Guppy (Poecilia reticulata), Plos One, 10, 2–18, 2015.
Zihler, F.: Gross morphology and configuration of digestive tracts of Cichlidae (Teleostei, Perciformes): phylogenetic and functional significance, Neth. J. Zool., 32, 544–571, 1982.
Zimmer, M.: Detritus. In: Encyclopedia of Ecology, 2nd Edn., vol. 3, edited by: Fath, B., 292–301, 2019.