the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Artificial perches increase bird-mediated seed rain in agricultural fallow area in southern Brazil
Thales Castilhos de Freitas
Gustavo Crizel Gomes
Artur Ramos Molina
Ernestino de Souza Gomes Guarino
Cristiano Agra Iserhard
Rafael Beltrame
One of the main barriers to restoration is the arrival of diaspores in degraded areas. However, this process can be hampered in open areas without trees in the landscape. For that, artificial perches are used to attract and provide a landing area for avian seed dispersers, to enhance seed rain. Our objective was to evaluate the effect of the distance of artificial perches in relation to a forest fragment on the diversity and composition of seed rain in an agricultural fallow area, including alien invasive plant species. We also aimed to record and characterize the bird species that potentially act as seed dispersers. Thus, we used artificial perches at three different distances from a forest fragment (5, 25, and 50 m). Four seed traps were arranged under the perches at each distance, and four control seed traps were interspersed with these and distanced at 7.5 m. Furthermore, we placed four seed traps inside the forest fragment at 5 m from the edge. We also carried out 80 h of focal observation of the avifauna that used artificial perches. A total of 24 655 seeds were sampled across all treatments. There was a significant difference in seed abundance and richness between artificial perches, control seed traps, and forest seed traps. Seed deposition increased with distance from the forest fragment (50, 25, and 5 m). An ordination procedure indicated the formation of three plant seed communities, with the forest community being most distinct. The invasive exotic species Pittosporum undulatum (Australian cheesewood) was the third most abundant in the seed rain. We observed 24 bird species from 12 families using artificial perches. The Tyrannidae family was the most represented. We showed that artificial perches are efficient structures for attracting birds, increasing the richness and abundance of seed species. Artificial perches at 25 and 50 m were more efficient possibly due to the provision of greater visibility for birds. Therefore, artificial perches are efficient in increasing seed rain in the fallow area but should be used with caution in landscapes with the presence of alien species. These findings contribute to increasing knowledge about overcoming the first barrier to ecological restoration, which is the arrival of diaspores in degraded areas, and showing the importance of birds in this process.
- Article
(3007 KB) - Full-text XML
- BibTeX
- EndNote
Overexploitation, habitat suppression, biological contamination, and climate change are accelerating species extinction processes and altering ecosystem services (Ceballos et al., 2017). Aligned with Sustainable Development Goal (SDG) 15, the UN defined the 2021–2030 decade as the Decade on Ecosystem Restoration, which aims to increase efforts to restore degraded ecosystems, creating efficient measures to fight against climate change, food production, conservation of water resources, and mitigation of biodiversity loss (Aronson et al., 2020).
To promote ecological restoration, nucleation is an important tool, being a set of techniques aimed at the formation of microhabitats, through structures or plantations in nuclei, facilitating the colonization of degraded areas by other plant species (Reis et al., 2014). The different techniques that make up nucleation have different objectives and approaches and may be related to soil restoration and the attraction of fauna or plant communities, thus being a complementary strategy to other restoration techniques, such as planting seedlings and natural regeneration (Bechara et al., 2021). In the case of artificial perches, the main objective is to overcome the first barrier to ecological restoration, which is the arrival of diaspores in the degraded area (Aide et al., 1995; Holl, 1999). According to Reid and Holl (2013), the arrival and deposition of diaspores are limiting processes in natural recovery. Thus, perches provide landing areas for frugivorous birds and bats to rest and forage and, through defecation and regurgitation, to deposit seeds under the perches, contributing to the formation of nuclei of diversity through allochthonous seed rain (Peña-Domene et al., 2014). The interaction between seed-dispersing plants and animals is essential in the formation of tropical forests (Carlo and Morales, 2016). For the deposition of seeds to arise, the occurrence of surrounding or nearby forest fragments is important as a food source for seed-dispersing animals (Brancalion et al., 2015; Carlo and Morales, 2016). The arrival of seeds by zoochoric dispersion can be difficult in open areas, especially if there are few or no trees remaining in the landscape, restricting this process only to the edges of natural fragments (Parrotta et al., 1997; Ponce et al., 2012). In addition, positioning artificial perches farther from the edge of forest fragments should be considered as this may affect bird activity and, consequently, seed deposition in the area to be restored (Nathan et al., 2003; Graham and Page, 2012).
Another important factor is the occurrence of invasive exotic plants in the landscape, mainly zoochoric, since this can result in the dispersion and deposition of these species seeds in the area under restoration (Pyšek et al., 2020). Several authors have already addressed the relationship between frugivory and seed dispersal of invasive alien species (Jordaan and Downs, 2012; Campagnoli et al., 2016; Ortega-Flores et al., 2018). The results have shown that the ingestion of seed species by birds improves the seed species' rate of germination (Freitas et al., 2020). This can promote the establishment of invasive plants that can interfere with and alter ecological processes, such as creating changes in nutrient cycling, biomass decomposition rates, plant community structure, pollination, seed dispersal, the aesthetic value of the landscape, and biodiversity loss due to species extinction (Ziller, 2001; Lourenço et al., 2011; Pyšek et al., 2020).
Therefore, the objective of this study is to evaluate the effect of the distance between artificial perches and a forest fragment on the diversity and composition of seed rain in an area under forest restoration, besides knowing the potentially dispersing avifauna. This study intends to answer the following questions: (1) does the use of artificial perches in the agricultural matrix increase the rain of seeds of zoochoric plants? (2) Does the distance of artificial perches from the forest fragment affect the composition and diversity of seed rain? (3) What is the contribution of alien invasive plants to the seed rain community under artificial perches? (4) Which bird species use artificial perches and act as potential seed dispersers?
2.1 Study area
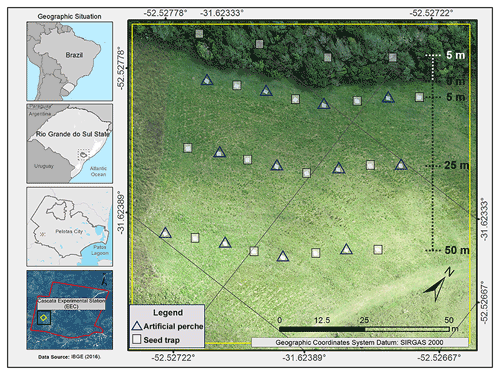
Our study was conducted at the Cascata Experimental Station (EEC) in Pelotas, Rio Grande do Sul, in southern Brazil, 160 According to the Köppen climate classification system, the region's climate is defined as Cfa, a humid temperate climate with hot summers, without a dry season (Alvares et al., 2013), and with a yearly average temperature of 18.9 ∘C and an average rainfall of 1794.6 mm. The study area has approximately 0.4 ha of fallow fields, previously covered by semi-deciduous seasonal forest, and is near a riparian forest (Fig. 1; IBGE, 2012). The riparian forest is characterized by native species such as Schinus terebinthifolia, Allophylus edulis, Myrsine coriacea, and Gymnanthes klotzschiana and alien species such as Pittosporum undulatum (Australian cheesewood), Pinus sp., Eucalyptus sp., and Hovenia dulcis. The landscape is composed of forest fragments of different sizes and regeneration stages and has been immersed in a consolidated agricultural matrix for more than a century.
2.2 Treatments
We made 12 artificial bamboo perches (4 m tall: 3.5 m above the ground and 0.5 m buried), with a “triple T” shape and three landing surfaces, 1 m long each and arranged at 1.5, 2.7, and 3.3 m from the ground surface (Fig. 2a). To evaluate seed rain, seed traps of 1 m2 were used, made with water-permeable fabric (anti-insect mesh), and placed 0.7 m from the soil (Fig. 2b). The perches (P) and control seed traps (C) were arranged in the fallow area, at three different distances from the edge of the forest fragment: 5 m (P5 m and C5 m), 25 m (P25 m and C25 m), and 50 m (P50 m and C50 m). At each distance, four seed traps were arranged under the perches, and four control seed traps were interspersed with these at 7.5 m from the perches. Furthermore, we placed four forest seed traps (F) at 5 m inside the edge of the forest fragment, at 14 m intervals (Fig. 2c).
2.3 Sampling
We conducted the experiment for 12 months (between 2017 and 2018). We performed the collection, screening, and identification of diaspores every 2 weeks, with a total of 24 samples per trap. For taxonomic identification, the collected material was compared (observation with the naked eye and stereomicroscope) with diaspores from the seed library of the Embrapa Clima Temperado and the Forest Sciences Laboratory of Faculdade de Agronomia at the Universidade Federal de Pelotas (UFPel), with the help of specialists and specialized bibliographic material (Lorenzi, 1992, 1998; Carvalho, 2003; 2006, 2008, 2010, 2014; Frigieri et al., 2016). In addition, we recorded all fruiting plant species dispersed by animals along transects at the edges and in the interior of nearby forest fragments during all four seasons. Plant species were classified based on the taxonomic system APG IV 2016 (Chase et al., 2016).
We observed the birds that used the artificial perches for 20 h per season per year (80 h of sampling effort) in four periods: dawn, late morning, early afternoon, and dusk. The focal observations were conducted with the aid of the Celestron binocular, Ultima model (8 × 42 magnification), at a distance of 20 m from the nearest perch, from a point where all perches were seen at the same time with no interference in bird activity. The visiting species and the length of stay on the perch were recorded for each visitation event. The taxonomic nomenclature of birds was based on the “Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee” (Piacentini et al., 2015).
2.4 Data analysis
We classified all seeds into zoochoric and non-zoochoric following the seed dispersal syndromes proposed by Van der Pijl (1982) and into native and alien species based on the “List of Invasive Alien Species of the State of Rio Grande do Sul” (SEMA, 2013) and the IABIN Invasives Information Network. The classification of birds regarding diet was based on Sick (1997) and on relevant bibliography (Francisco and Galetti, 2002; Pizo, 2004; Jesus and Monteiro-Filho, 2007; Tubelis, 2007; Pascotto, 2007; Howe, 2017).
To assess the richness and abundance of seeds, we fitted a generalized linear mixed model (GLMM), using the individual samples of each treatment. As these variables correspond to counts (i.e., number of species and number of seeds, and to account for overdispersion in the models residuals, we fitted negative binomial models as they showed better fits and lower deviance than Poisson models (Zuur et al., 2009). We fitted two different models for each response variable: one model considering the treatment (control seed traps, forest seed traps, and artificial perches) as a fixed variable and one model considering the distance of forest seed traps (5 m) and the distinct distances between perches and the forest fragment as a fixed variable. In order to account for non-independence of repeated samples from the same seed trap, we considered each seed trap as a random effect in the GLMMs. We plotted the values of richness and abundance of seeds in artificial perches, forest seed traps, control seed traps, and the distinct distances of perches (5, 25, and 50 m) in box plots. After, we used multiple comparisons based on a GLMM (negative binomial model, considering seed traps as a random effect) to compare richness and abundance among these treatments. These analyses were conducted in the R environment (R Core Team, 2022) using functions from the lme4 package (Bates et al., 2015).
To evaluate the differences in the composition of seeds deposited in artificial perches and inside the forest fragment, a non-metric multidimensional scaling (NMDS) was performed using the Bray–Curtis quantitative dissimilarity index (IsBC) (Magurran, 2013). The fit of the NMDS ordinations was quantified by a value of stress. In addition, to test for differences between seed species groupings obtained with the NMDS, a multivariate permutational variance analysis (PERMANOVA) was performed using the same similarity measurement and 999 randomizations (PAST 3.20 software; Hammer, 2001).
For the distinct distances between perches and the forest fragment, the diversity profile was calculated using the estimated model through the Chao1 index with 1000 repetitions, in which the overlap between the confidence intervals of the communities indicates the absence of significant difference (Chao and Jost, 2012). The diversity profile is represented by species richness (Q0) without considering their relative abundances, the exponential of Shannon entropy (Q1), Simpson's dominance index (Q2), and the Berger–Parker index (Q3). The more Q increases, the higher the value given to the dominant species (Hill, 1973; Gotelli and Chao, 2013; de Vries et al., 2021). The iNEXT software was used to calculate the diversity profile (Chao et al., 2016).
3.1 Seed rain
A total of 24 655 seeds were sampled, distributed in 23 families and 34 species of plants. For 6 taxa, it was possible to reach only the family or genus, in addition to 11 morphospecies, totaling 51 different diaspores (Appendix A). For morphospecies, it was not possible to identify their dispersal syndrome. The most abundant species in the seed rain were Solanum americanum (n=9794), Ficus organensis (2993), Pittosporum undulatum (2835), and Schinus terebinthifolia (2157).
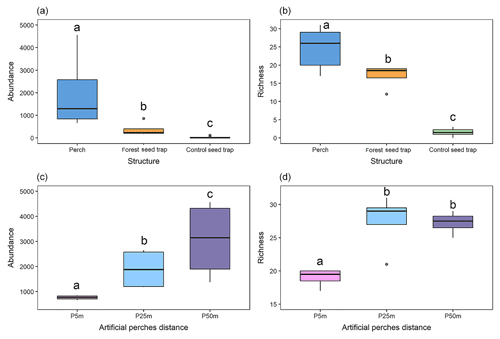
Figure 3Graphical representation of the variation in (a) the abundance of seed rain under the different structures; (b) seed rain richness under the different structures; (c) the abundance of seed rain under artificial perches at 5, 25, and 50 m from the edge of the forest fragment; and (d) richness of seed rain under artificial perches at 5, 25, and 50 m from the edge of the forest fragment. Significant differences between groups at p < 0.05 are denoted by different letters.
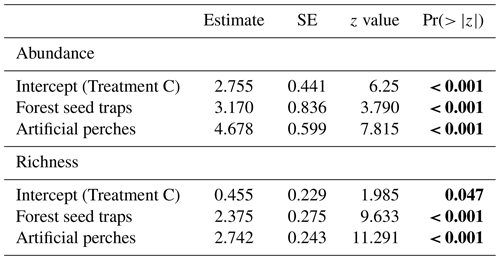
Under the artificial perches (P), 22 892 seeds were deposited (mean ± SD, 1907.25 ± 1347.53) with a richness of 46 species (24.58 ± 4.87); in the control seed traps (C), 256 seeds (21 ± 34.23) of 5 species (1.58 ± 1.08) were deposited; and in the forest seed traps (F), 1507 seeds (374.5 ± 323.04) of 29 species (17 ± 3.36) were deposited. According to GLMM analysis, there was a significant difference in seed abundance and richness between control seed traps (C), forest seed traps, and artificial perches (Table 1 and Fig. 3a and b). For both response variables, higher values occurred in artificial perches, followed by forest seed traps and control seed traps.
Table 1GLMM output from effects of treatments on abundance and richness of seeds sampled at the Cascata Experimental Station, southern Brazil. SE: standard error; Treatment C: control seed traps. The values are on the log-link scale. Values in bold indicate significant differences.
Regarding the seed rain, the GLMM indicates significant differences between forest seed traps and perches at different distances to the forest fragment and among perches at all distances (Table 2 and Fig. 3c). The largest seed deposition occurred at a distance of 50 m (3060 ± 1575) from the forest fragment, followed by 25 m (1897 ± 813) and 5 m (765 ± 86). Considering richness among artificial perches at different distances and seed traps in the forest fragment, the GLMM showed a significant difference between forest seed traps and perches at 25 and 50 m but not with perches at 5 m (Table 2). Richness among artificial perches was significantly different between perches at 5 m and perches at 25 and 50 m, which showed no significant differences (Fig. 3d). Perches at 25 m from the forest edge had the highest number of species (27.50 ± 4.34), followed by perches at 50 m (27.25 ± 1.70) and by perches at 5 m (19 ± 1.41).
Table 2GLMM output from effects of forest seed traps and distances of artificial perches (5, 25, and 50 m) on abundance and richness of seeds sampled at the Cascata Experimental Station, southern Brazil. SE: standard error. The values are on the log-link scale. Values in bold are significant.
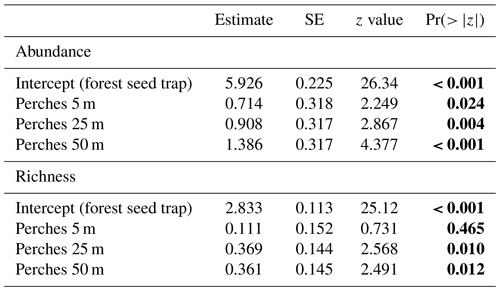
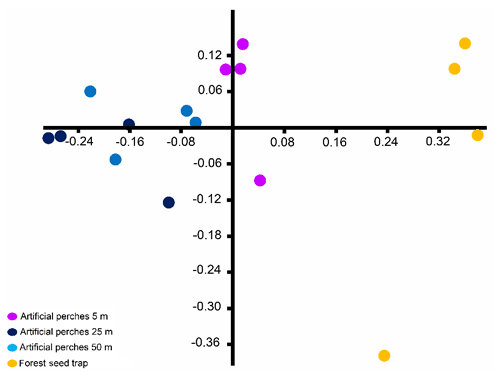
Figure 4Non-metric multidimensional scaling (stress: 0.076) ordination based on Bray–Curtis dissimilarity of seed community deposited under artificial perches and forest seed traps sampled at the Cascata Experimental Station, southern Brazil.
Table 3One-way statistical test PERMANOVA, concerning the composition of seed rain in perches (5, 25, and 50 m) and forest seed traps, at different distances (p < 0.05).

∗ Significant difference.
The NMDS indicates segregation in the composition of seed species between the forest seed traps and the different distances of artificial perches in the fallow area and that perches at 25 and 50 m have greater similarity in the seed species deposited under these structures (Fig. 4). The overall result of PERMANOVA shows differences (pseudo-; p=0.001) in the similarity of seeds between the forest seed species and artificial perches. In this way, the forest seed traps had a distinct species composition when compared to all perches' distances, and among perches there were significant differences between perches at 5 m when compared with 25 and 50 m, which were similar to each other (Table 3).
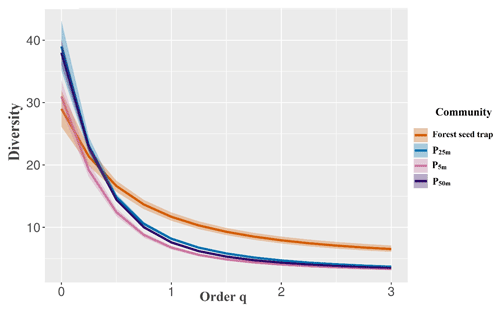
Figure 5Diversity profile of perches at 5, 25, and 50 m from the edge of the forest fragment and forest seed traps sampled at the Cascata Experimental Station, southern Brazil.
There was no significant difference in the diversity profile between the estimated richness (q=0) due to the overlap of the intervals. The forest seed traps presented higher equitability and lower dominance (q1, q2, and q3), indicating a greater diversity of the plant community in the forest area compared to the other treatments (Fig. 5), which were similar between the different distances. The high dominance of some species in the seed rain was observed in artificial perches, in which 79 % of the seeds were restricted to five species in the perches at 50 m (Solanum americanum, Ficus organensis, Pittosporum undulatum, Myrsine spp. 1, and Schinus terebinthifolia), and 71 % of the seeds came from only three species, a fact that is repeated for the perches at 5 m (Solanum americanum, Schinus terebinthifolia, and Pittosporum undulatum). The most abundant species for the perches at 25 m were Solanum americanum, Pittosporum undulatum, and Schinus terebinthifolia.
There were two invasive plant species in the experiment, namely Pittosporum undulatum (2835 seeds) and Lonicera japonica (61 seeds). Pittosporum undulatum represented 11.49 % of the total seed rain in the experiment, occurring at all distances and structures, with higher deposition on perches at 50 m. As a climber plant, Lonicera japonica occurred predominantly in the forest (96 %) and was absent in control seed traps.
3.2 Bird community
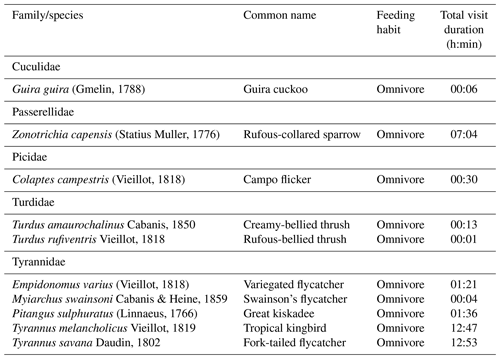
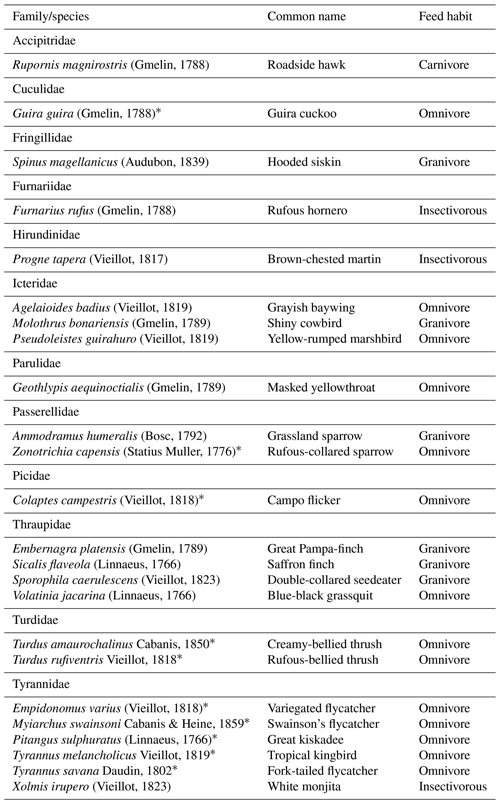
We observed 24 species from 12 bird families using artificial perches during the experiment, and several eating habits were recognized (Appendix B). Of this total, 10 are potentially seed dispersers (Table 4). Of the 80 h of focal observation in artificial perches, 45.85 % of the time at least one bird was using the structures, corresponding to 36 h and 41 min of use. Of this total, the species Tyrannus savanna, T. melancholicus, Zonotrichia capensis, Pitangus sulphuratus, and Empidonomus varius represented 97 % of the length of stay on the structures, with Pitangus sulphuratus being the only species that used perches throughout the year.
The Tyrannidae family was most frequently observed during the experiment. Five of the six species that used the structures are considered seed dispersers. Tyrannus savanna and T. melancholicus occurred in the experiment only in spring and summer as they are migratory birds to the region (Timm and Timm, 2016).
4.1 Seed rain
We showed that artificial perches are efficient structures for attracting seed dispersers, increasing the richness and abundance of seed species (≈ 90× more seeds in artificial perches) at least up to a distance of 50 m from the forest fragment. The diversity of seeds is higher inside the forest fragment, and the species composition in the seed rain differed between the forest fragment and perches but was similar between perches at 25 and 50 m from the edge of the forest.
The farther the artificial perches were from the forest fragment edge, the more abundant and richer in species the seed rain was. These increasing values of richness and abundance of seeds may be related to the landscape features that provide several feeding resources to the birds with generalist habits that used the perches to move across the landscape. Distinct landscape elements, such as isolated trees, riparian forests, and forest patches in different successional stages, can enhance the dispersal of seeds (Zwiener et al., 2014). During the study, we observed the movement of birds to the perches, mainly crossing the open areas instead of using the closer forest fragment, although some birds accessed the fragment after having used the artificial perches. This pattern probably contributed to the greater abundance of seeds in the more distant perches embedded in the open matrix of the studied area. In addition, the availability of landing structures in fields favors the attraction of birds and the increase in seed deposition on the site, promoting a greater seed rain in these places than in open areas because these structures provide shelter, rest, and foraging for birds (Holl, 1998; Graham and Page, 2012; Alencar and Guilherme, 2020). Thus, perches function as stepping stones, connecting close fragments that favor gene flow (Sant'anna, 2011; Pustkowiak et al., 2021).
Nevertheless, the efficiency of seed rain at distinct distances from the forest edge encompasses several factors like (i) fruit and seed availability, (ii) the presence of forest fragments and isolated trees in the landscape, (iii) which seed dispersers there are in the region, (iv) the distribution of perches in the landscape, and (v) visual acuity of the dispersers (Brancalion et al., 2015; Almeida et al., 2016; Carlo and Morales, 2016). Our results corroborate studies that evaluate the use of perches across different distances (Alencar and Guilherme, 2020) but contrast with other studies that did find differences between perches near or far from forest fragments (Dias et al., 2014; Iguatemy et al., 2020). One study (Zwiener et al., 2014) found differences for plant species richness but not for abundance among different perch distances. This shows that there is no marked pattern for this kind of study; results instead depend on a set of factors, like spatial scale evaluated, type of ecosystem, and the biotic and abiotic variables analyzed. However, the use of artificial perches is effective to overcome the barrier of seed arrival in open habitats compared to studies without structures to attract fauna (Cubiña and Aide, 2001; Piotto et al., 2019).
When we consider the equitability of the species, beyond richness and abundance, the artificial perches at farther distances presented high dominance despite presenting a higher number of seeds and species reflecting a low diversity compared to seed traps in the forest fragment, which shows a greater diversity. The forest fragment presents a more heterogeneous structure, with several ecological niches and with greater diversity of zoochoric birds, which do not use artificial perches but fulfill their role of dispersers within the forest fragment. In tropical forests, up to 90 % of plants depend on birds to be dispersed, thus forming diverse and complex seed dispersal networks characterized by a large number of bird species and interactions with plants (Emer et al., 2020). Artificial perches may act as a filter for the arrival of diaspores because the shape, ramifications, and heights of these structures will influence differently their use by distinct bird species that disperse small seeds (McClanahan and Wolfe, 1987; Holl, 1998). Another factor that limits the efficiency of this technique is seed predation by rodents, insects, and pathogens, decreasing the rates of seedling emergence in open areas, as well as soil compaction and competition with grasses to establish saplings (Holl et al., 2000; Almeida et al., 2016).
On the other hand, the low richness and high dominance by generalist birds, which were observed using artificial perches, are relatively expectable because few species can disperse seeds in both open and forested areas (Pizo and Santos, 2011). In addition, the high representativeness of some plant species in artificial perches may be due to the large number of fruits and seeds that these plants provide, their small fruit size, and visibility, besides harvest season and the time in which they are available for the bird fauna (Jesus and Monteiro-Filho, 2007; Pascotto, 2007). This is the case for Schinus terebinthifolia, Ficus organensis, Pittosporum undulatum, and Solanum americanum, which are abundant and common species in the landscape and at the edge of the fragments and present long fruiting periods, being important sources of food resources in times of scarcity, such as in winter (Jesus and Monteiro-Filho, 2007; Vissoto et al., 2019).
This dominance of dispersing birds and seeds contributed to the formation of distinct plant communities in the study area. The forest seed trap community possibly differed from the others due to the composition of plants and dispersers being different from artificial perches in the open area. Forest specialist birds rarely leave forest fragments, and the few that go into open areas do it for short periods of time (Da Silva et al., 1996). No forest specialist birds were observed using the perches in the open area, probably because the birds prefer natural perches, using the edge trees and depositing their seeds in these places. This fact was corroborated in our study due to differences in richness and abundance and due to the formation of different seed species communities. However, the forest seed community had greater diversity.
Seed rain abundance and richness show the same general pattern, with more distant perches having higher values than perches at 5 m and forest seed traps. This pattern is even more marked for the abundance of seeds because all distances were different to each other, but in this case artificial perches at 50 m had more deposited seeds. The different seed compositions between the perches near and far from the forest fragment depend on several factors, such as the surrounding landscape with the source of propagules and resources for birds species (both in quality and in quantity), the dispersing birds, and the distance from artificial perches perceived by birds (Dias et al., 2014; Zwiener et al., 2014; Alencar and Guilherme, 2020; Iguatemy et al., 2020).
We emphasize the large seed deposition of Pittosporum undulatum, an invasive alien species of Australian origin (Goodland and Healey, 1996; IABIN-I3N, 2019), demonstrating a negative aspect of the use of artificial perches in landscapes with the presence of invasive plant species, since there is no selectivity of seeds deposited by the avifauna. The danger of biological invasion in restoration programs is worrisome, as they prevent ecological succession and tend to be highly aggressive (Tomazi et al., 2010). Pittosporum undulatum is an abundant species in the studied region with a density of 1492 individuals ha−1 in nearby forest fragments (Karam and Cardoso, 2010), besides several isolated individuals in the landscape. This expressive bioinvasion is reflected in the reproduction ability of the species with about 20 and 40 seeds per fruit (Goodland and Healey, 1996) and with a long fructification period, mainly in periods of scarcity of other fruit resources (the seeds were recorded across 8 consecutive months, March to October, in the artificial perches). Pitangus sulphuratus, an abundant and generalist bird species found in the region, is an important agent in the dispersion and invasion of Pittosporum undulatum, having a positive effect on the germination of this species after the defecation (Freitas et al., 2020). In addition, the genus Turdus is considered another disperser of Pittosporum undulatum in the Atlantic Forest (Campagnoli et al., 2016). Thus, some birds prefer to feed on exotic species instead of native species (Mokotjomela et al., 2013; Maruyama et al., 2016).
Other studies using artificial perches also found exotic plant species in the seed rain, as did Marcuzzo et al. (2013) in the restoration of urban fragments, with the presence of Melia azedarach and Psidium guajava being attributed to the high presence of these species in urban afforestation. The deposition of Morus nigra and P. guajava was observed under artificial perches in an ecological restoration project in the Atlantic Forest (Almeida et al., 2016). Due to the high abundance of Pittosporum undulatum seeds (the third largest in the present study), care should be taken in the use of artificial perches as an ecological restoration technique in this region. Artificial perches can be replaced by other techniques (i.e., plant mat transplantation, islets of seedlings, direct seeding, and topsoil seed banks of preserved forest fragments) that will not promote the dispersion of invasive species; otherwise the situation requires constant monitoring and control of seeds or seedlings of this species and other invasive alien species that may arise during restoration.
4.2 Bird community
All species observed using artificial perches have generalist habits for both habitat and feed (Sick, 1997; Francisco and Galetti, 2002; Pizo, 2004; Jesus and Monteiro-Filho, 2007; Howe, 2017). This group of birds has great importance in ecological restoration, especially in the dispersion of seeds over long distances, because the birds have a great capacity to explore many resources (González-Castro et al., 2019; Camargo et al., 2020; Campos-Silva and Piratelli, 2021).
Among generalist birds, the Tyrannidae family stands out, representing almost the total length of permanence in the artificial perches, using these structures for rest and foraging and having an overview of the area to search for food. Tyranids are able to eat fruits, poultry, and also insects, foraging in forest edges and more open areas and being able to disperse seeds from forest fragments into open habitats (Guedes et al., 1997; Timm and Timm, 2016). Thus, it is possible that they had contributed with seeds from other forest fragments and isolated trees in the landscape. This behavior was observed, mainly, for T. savanna, T. melancholicus, Pitangus sulphuratus, and E. varius, which captured insects in mid-flight and returned to the perches, defecating under the structures during rest (personal observation). Several studies using artificial perches have reported the presence of generalist species and have attributed the high rate of seed deposition to the family Tyrannidae (Guedes et al., 1997; Bocchese et al., 2008; Athiê and Dias, 2016). According to Camargo et al. (2020), the species Pitangus sulphuratus and T. melancholicus accounted for almost half of all bird activity in the ecological restoration experiment.
In this work we aimed to understand the effectiveness of artificial perches as a restoration technique in assisting the dispersal of seeds by birds into degraded areas. Future studies will further benefit from carrying out bird census inside the forest fragment to compare with the community of birds using artificial perches, as well as assessing the use of perches at different distance by distinct bird species. Furthermore, following germination and seedling recruitment success, as well as their relationship with seed predation in open areas (Bocchese et al., 2008), will allow a better assessment of the restoration success of artificial perches. Finally, using more than one nucleation technique and increasing replication will also allow us to reach more robust results.
We showed that artificial perches are effective structures to increase seed rain in open areas. We found that birds from the Tyrannidae family make the greatest contribution to the restoration of this degraded agricultural landscape. The diaspores come not only from the nearest forest fragment but also from trees scattered in the landscape or at the edge of the fragments, thus providing a more diverse and heterogeneous seed rain and contributing to breaking the first barrier to ecological restoration, which is the arrival of diaspores in the degraded area. Nonetheless, the use of artificial perches in ecological restoration should be used with caution due to the potential to contribute to the dispersion of seeds and expansion of invasive plant species. This calls for the use of other restoration techniques or the specific monitoring of such species.
The data used in this study are provided in Appendix A and Appendix B.
TCdF, GCG, and EdSGG conceived and designed the experiments. All authors performed field work, wrote the paper, and approved the final manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors are thankful to Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for the resources provided to PPGCAmb, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; proc. 441575/2017-0).
This research has been supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (grant no. 001).
This paper was edited by Sérgio Timóteo and reviewed by three anonymous referees.
Aide, T. M., Zimmerman, J. K., Herrera, L., Rosario, M., and Serrano, M.: Forest recovery in abandoned tropical pastures in Puerto Rico, Forest Ecol. Manag., 77, 77–86, https://doi.org/10.1016/0378-1127(95)03576-V, 1995.
Alencar, L. and Guilherme, E.: Artificial perches for the supply of seeds in a fragmented landscape in southwest Brazilian Amazon, Rev. Bras. Bot., 43, 1013–1023, https://doi.org/10.1007/s40415-020-00662-z, 2020.
Almeida, A., Marques, M. C. M, Ceccon-Valente, F. M., Silva, J. V., and Mikich, S. B.: Limited effectiveness of artificial bird perches for the establishment of seedlings and the restoration of Brazil's Atlantic Forest, J. Nat. Conserv., 34, 24–32, https://doi.org/10.1016/j.jnc.2016.08.007, 2016.
Alvares, C. A., Stape, J. L., Sentelhas, P. C., Gonçalves, J. L. M., and Sparovek, P. C.: Koppen's climate classification map for Brazil, Meteorol. Z., 22, 711–728, https://doi.org/10.1127/0941-2948/2013/0507, 2013.
Aronson, J., Goodwin, N., Orlando, L., Eisenberg, C., and Cross, A. T.: A world of possibilities: six restoration strategies to support the United Nation's Decade on Ecosystem Restoration, Restor. Ecol., 28, 730–736, https://doi.org/10.1111/rec.13170, 2020.
Athiê, S. and Dias, M. M.: Use of perches and seed dispersal by birds in an abandoned pasture in the Porto Ferreira state park, southeastern Brazil, Braz. J. Biol., 76, 80–92, https://doi.org/10.1590/1519-6984.13114, 2016.
Bates, D., Mächler, M., Bolker, B., and Walker, S.: Fitting Linear Mixed-Effects models using lme4, J. Stat. Softw., 67, 1–48, https://doi.org/10.18637/jss.v067.i01, 2015.
Bechara, F. C., Trentin, B. E., Engel, V. L., Estevan, D. A., and Ticktin, T.: Performance and cost of applied nucleation versus high-diversity plantations for tropical forest restoration, Forest Ecol. Manage., 491, 119088, https://doi.org/10.1016/j.foreco.2021.119088, 2021.
Bocchese, R. A., Oliveira, A. D., Favero, S., Garnés, S. D. S., and Laura, V. A.: Chuva de sementes e estabelecimento de plântulas a partir da utilização de árvores isoladas e poleiros artificiais por aves dispersoras de sementes, em área de Cerrado, Mato Grosso do Sul, Brasil, Rev. Bras. Ornitol., 16, 207–213, 2008.
Brancalion, P. H., Gandolfi, S., and Rodrigues, R. R.: Restauração Florestal, Oficina de Textos, São Paulo, ISBN 978-85-7975-019-9, 2015.
Camargo, P. H., Pizo, M. A., Brancalion, P. H., and Carlo, T. A.: Fruit traits of pioneer trees structure seed dispersal across distances on tropical deforested landscapes: Implications for restoration, J. Appl. Ecol., 57, 2329–2339, https://doi.org/10.1111/1365-2664.13697, 2020.
Campagnoli, M. L., Santos, S. R. G., Silva, S. D. S. R., and Antunes, A. Z.: O papel das aves na dispersão e germinação de sementes do pau-incenso (Pittosporum undulatum Vent.) em um remanescente de Mata Atlântica, Rev. do Inst Florest., 28, 59–67, 2016.
Campos-Silva, L. A. and Piratelli, A. J.: Vegetation structure drives taxonomic diversity and functional traits of birds in urban private native forest fragments, Urban Ecosyst., 24, 375–390, https://doi.org/10.1007/s11252-020-01045-8, 2021.
Carlo, T. A. and Morales, J. M.: Generalist birds promote tropical forest regeneration and increase plant diversity via rare-biased seed dispersal, Ecology, 97, 1819–1831, https://doi.org/10.1890/15-2147.1, 2016.
Carvalho, P. E. R.: Espécies arbóreas nativas, vol. 1, Embrapa Informação Tecnológica, Brasília, ISBN 85-7383-167-7, 2003.
Carvalho, P. E. R.: Espécies arbóreas brasileiras, vol. 2, Embrapa Informação Tecnológica, Brasília, ISBN 85-7383-373-4, 2006.
Carvalho, P. E. R.: Espécies arbóreas brasileiras, vol. 3, Embrapa Informação Tecnológica, Brasília, ISBN 978-85-7383-429-1, 2008.
Carvalho, P. E. R.: Espécies arbóreas brasileiras, vol. 4, Embrapa Informação Tecnológica, Brasília, ISBN 978-85-7383-487-1, 2010.
Carvalho, P. E. R.: Espécies arbóreas brasileiras, vol. 5, Embrapa Informação Tecnológica, Brasília, ISBN 978-85-7035-338-2, 2014.
Ceballos, G., Ehrlich, P. R., and Dirzo, R.: Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines, P. Natl. Acad. Sci. USA, 114, 6089–6096, https://doi.org/10.1073/pnas.1704949114, 2017.
Chao, A. and Jost, L.: Diversity measures, University of California Press, https://doi.org/10.1525/9780520951785-040, 2012.
Chao, A., Ma, K. H., and Hsieh, T. C.: iNEXT (iNterpolation and EXTrapolation), Software for Interpolation and Extrapolation of Species Diversity, http://chao.stat.nthu.edu.tw/wordpress/software_download/ (last access: 20 August 2020), 2016.
Chase, M. W., Christenhusz, M. J. M., Fay, M. F., Byng, J. W., Judd, W. S., Soltis, D. E., and Stevens, P. F.: An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV, Bot. J. Linn. Soc., 181, 1–20, 2016.
Cubiña, A. and Aide, T. M.: The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture, Biotropica, 33, 260–267, https://doi.org/10.1111/j.1744-7429.2001.tb00177.x, 2001.
Da Silva, J. M. C., Uhl, C., and Murray, G.: Plant succession, landscape management, and the ecology of frugivorous birds in abandoned Amazonian pastures, Conserv. Biol., 10, 491–503, https://doi.org/10.1046/j.1523-1739.1996.10020491.x, 1996.
De Vries, J. P. R., van Loon, E., and Borges, P. A. V.: A Small-Scale Analysis of Elevational Species Richness and Beta Diversity Patterns of Arthropods on an Oceanic Island (Terceira, Azores), Insects, 12, 936, https://doi.org/10.3390/insects12100936, 2021.
Dias C. R., Umetsu, F., and Breier, T. B.: Contribuição dos poleiros artificiais na dispersão de sementes e sua aplicação na restauração florestal, Cienc. Florest., 24, 501–507, https://doi.org/10.5902/1980509814590, 2014.
Emer, C., Jordano, P., Pizo, M. A., Ribeiro, M. C., da Silva, F. R., and Galetti, M.: Seed dispersal networks in tropical forest fragments: Area effects, remnant species, and interaction diversity, Biotropica, 52, 81–89, https://doi.org/10.1111/btp.12738, 2020.
Francisco, M. R. and Galetti, M.: Aves como potenciais dispersoras de sementes de Ocotea pulchella Mart (Lauraceae) numa área de vegetação de cerrado do sudeste brasileiro, Rev. Bras. Bot., 25, 11–17, 2002.
Freitas, T. C., Guarino, E. D. S. G., Gomes, G. C., Molina, A. R., da Luz Real, I. M., and Beltrame, R.: The effect of seed ingestion by a native, generalist bird on the germination of worldwide potentially invasive trees species Pittosporum undulatum and Schinus terebinthifolia, Acta Oecol., 108, 1–6, https://doi.org/10.1016/j.actao.2020.103639, 2020.
Frigieri, F. F., Iwanicki, N. S., Gandara, F. B., Ferraz, E. M., Romão, G. O., Coletti, G. F., Souza, V. C., and Moreno, M. A.: Guia de plântulas e sementes da Mata Atlântica do estado de São Paulo, IPEF, Piracicaba, ISBN 978-85-89142-06-9, 2016.
González-Castro, A., Yang, S., and Carlo, T. A.: How does avian seed dispersal shape the structure of early successional tropical forests?, Funct. Ecol., 33, 229–238, https://doi.org/10.1111/1365-2435.13250, 2019.
Goodland, T. and Healey, J. R.: The invasion of Jamaican rainforests by the Australian tree Pittosporum undulatum, University of Wales, Bangor, https://www.researchgate.net/publication/260592247_The_invasion_of_Jamaican_montane_rainforests_by_the_Australian_tree_Pittosporum_undulatum/link/5630e1ec08ae13bc6c35412e/download (last access: 30 October 2022), 1996.
Gotelli, N. J. and Chao, A.: Measuring and estimating species richness, species diversity, and bioticsimilarity from sampling data, Encyclopedia of Biodiversity, Waltham, https://doi.org/10.1016/B978-0-12-384719-5.00424-X, 2013.
Graham, L. L. and Page, S. E.: Artificial bird perches for the regeneration of degraded tropical peat swamp forest: a restoration tool with limited potential. Restor. Ecol., 20, 631–637, https://doi.org/10.1111/j.1526-100X.2011.00805.x, 2012.
Guedes, M. C., Melo, V. A., and Griffith, J. J.: Uso de poleiros artificiais e ilhas de vegetação por aves dispersoras de sementes, Ararajuba, 5, 229–232, 1997.
Hammer, Ø., Harper, D. A., and Ryan, P. D.: PAST: Paleontological statistics software package for education and data analysis, Palaeontol. Electron., 4, 1–9, 2001.
Hill, M. O.: Diversity and evenness: a unifying notation and its consequences, Ecology, 54, 427–432, https://doi.org/10.2307/1934352, 1973.
Holl, K. D.: Do bird perching structures elevate seed rain and seedling establishment in abandoned tropical pasture?, Restor. Ecol., 6, 253–261, https://doi.org/10.1046/j.1526-100X.1998.00638.x, 1998.
Holl, K. D.: Factors limiting tropical rain forest regeneration in abandoned pasture: Seed rain, seed germination, microclimate, and soil, bioTropica, 31, 229–242, https://doi.org/10.1111/j.1744-7429.1999.tb00135.x, 1999.
Holl, K. D., Loik, M. E., Lin, E. H., and Samuels, I. A.: Tropical montane forest restoration in Costa Rica: overcoming barriers to dispersal and establishment, Restor. Ecol., 8, 339–349, https://doi.org/10.1046/j.1526-100x.2000.80049.x, 2000.
Howe, H. F.: Fruit-eating birds in experimental plantings in southern Mexico, J. Trop. Ecol., 33, 83–88, https://doi.org/10.1017/S0266467416000596, 2017.
IABIN-I3N: Inter-American Biodiversity Information Network, https://bd.institutohorus.org.br/especies, last access: 3 April 2019.
IBGE: Manual técnico da vegetação brasileira, Série manuais técnicos em Geociências 1, 2a ed., Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro, ISBN 978-85-240-4272-0, 2012.
Iguatemy, M. D. A., Vilarinhos, J. A., Oda, G. A. M., Conde, M. D. M. S., and Zaú, A. S.: Artificial perches: ecological and functional aspects of its contribution in the Atlantic forest, Floresta e Ambiente, 27, 1–8, https://doi.org/10.1590/2179-8087.030118, 2020.
Jesus, S. and Monteiro-Filho, A. E. L.: Frugivory by birds in Schinus terebinthifolius (Anacardiaceae) and Myrsine coriacea (Myrsinaceae), Rev. Bras. Ornitol., 15, 585–591, 2007.
Jordaan, L. A. and Downs, C. T.: Comparison of germination rates and fruit traits of indigenous Solanum giganteum and invasive Solanum mauritianum in South Africa, S. Afr. J. Bot., 80, 13–20, https://doi.org/10.1016/j.sajb.2012.01.007, 2012.
Karam, L. D. M. and Cardoso, J. H.: Caracterização fitossociológica do impacto de Pittosporum undutalum Vent. em três Fragmentos de Floresta Estacional Semidecidual (FESD) na encosta da Serra do Sudeste, Pelotas, RS, Bol. Pesq. Desenv., 127, Embrapa Clima Temperado , p. 20, ISSN 1678-2518, 2010.
Lorenzi, H.: Árvores brasileiras, vol. 1, Editora Plantarum, São Paulo, ISBN 978-65-87655-00-0, 1992.
Lorenzi, H.: Árvores brasileiras, vol. 2. Editora Plantarum, São Paulo, ISBN 978-65-87655-07-9, 1998.
Lourenço, P., Medeiros, V., Gil, A., and Silva, L.: Distribution, habitat and biomass of Pittosporum undulatum, the most important woody plant invader in the Azores Archipelago. Forest Ecol. Manag., 262, 178–187, https://doi.org/10.1016/j.foreco.2011.03.021, 2011.
Magurran, A. E.: Medindo a diversidade biológica, Editora UFPR, Curitiba, ISBN 978-85-7335-278-8, 2013.
Marcuzzo, S. B., Ganade, G., Araújo, M. M., and Muniz, M. F. B.: Comparação da eficácia de técnicas de nucleação para restauração de área degradada no sul do Brasil, Floresta, 43, 39–48, https://doi.org/10.5380/rf.v43i1.28680, 2013.
Maruyama, P. K., Vizentin-Bugoni, J., Sonne, J., Martin Gonzalez, A. M., Schleuning, M., Araujo, A. C., and Dalsgaard, B.: The integration of alien plants in mutualistic plant–hummingbird networks across the Americas: the importance of species traits and insularity, Divers. Distrib., 22, 672–681, https://doi.org/10.1111/ddi.12434, 2016.
McClanahan, T. R. and Wolfe, R. W.: Dispersal of ornithochorous seeds from forest edges in central Florida, Vegetatio, 71, 107–112, 1987.
Mokotjomela, T. M., Musil, C. F., and Esler, K. J.: Frugivorous birds visit fruits of emerging alien shrub species more frequently than those of native shrub species in the South African Mediterranean climate region, S. Afr. J. Bot., 86, 73–78, https://doi.org/10.1016/j.sajb.2013.02.004, 2013.
Nathan, R., Perry, G., Cronin, J. T., Strand, A. E., and Cain, M. L.: Methods for estimating long-distance dispersal, Oikos, 103, 261–273, https://doi.org/10.1034/j.1600-0706.2003.12146.x, 2003.
Ortega-Flores, M., Maya-Elizarrarás, E., and Schondube, J. E.: Effects of Rufous-Backed Robin (Turdus rufopalliatus) on Brazilian Pepper-Tree (Schinus terebinthifolius) Seed Germination and Dispersal in a Subtropical Peri-Urban Environment, Trop. Conserv. Sci., 11, 1–8, https://doi.org/10.1177/1940082918761022, 2018.
Parrotta, J. A., Turnbull, J. W., and Jones, N.: Catalyzing native forest regeneration on degraded tropical lands. Forest Ecol. Manag., 99, 1–7, https://doi.org/10.1016/S0378-1127(97)00190-4, 1997.
Pascotto, M. C.: Rapanea ferruginea (Ruiz & Pav.) Mez. (Myrsinaceae) como importante fonte alimentar para as aves em uma mata de galeria no interior do Estado de São Paulo, Rev. Bras. Zool., 24, 735–741, https://doi.org/10.1590/S0101-81752007000300026, 2007.
Peña-Domene, M., Martinez-Garza, C., Palmas-Perez, S., Rivas-Alonso, E., and Howe, H. F.: Roles of birds and bats in early tropical-forest restoration, PLOS ONE, 9, e104656, https://doi.org/10.1371/journal.pone.0104656, 2014.
Piacentini, V. D. Q., Aleixo, A., Agne, C. E., Maurício, G. N., Pacheco, J. F., Bravo, G. A., and Silveira, L. F.: Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee, Rev. Bras. Ornitol., 23, 90–298, https://doi.org/10.1007/BF03544294, 2015.
Piotto, D., Craven, D., Montagnini, F., Ashton, M., Oliver, C., and Thomas, W. W.: Successional, spatial, and seasonal changes in seed rain in the Atlantic forest of southern Bahia, Brazil, PLOS ONE, 14, e0226474, https://doi.org/10.1371/journal.pone.0226474, 2019.
Pizo, M. A.: Frugivory and habitat use by fruit-eating birds in a fragmented landscape of southeast Brazil, Ornitol. Neotrop., 15, 117–126, 2004.
Pizo, M. A. and dos Santos, B. T. Frugivory, post-feeding flights of frugivorous birds and the movement of seeds in a Brazilian fragmented landscape. Biotropica, 43, 335–342, https://doi.org/10.1111/j.1744-7429.2010.00695.x, 2011.
Ponce, A. M., Grilli, G., and Galetto, L.: Frugivoría y remoción de frutos ornitócoros en fragmentos del bosque chaqueño de Córdoba (Argentina), Bosque, 33, 33–41, https://doi.org/10.4067/S0717-92002012000100004, 2012.
Pustkowiak, S., Kwieciński, Z., Lenda, M., Żmihorski, M., Rosin, Z. M., Tryjanowski, P., and Skórka, P.: Small things are important: the value of singular point elements for birds in agricultural landscapes, Biol. Rev., 96, 1386–1403, https://doi.org/10.1111/brv.12707, 2021.
Pyšek, P., Hulme, P. E., Simberloff, D., Bacher, S., Blackburn, T. M., Carlton, J. T., and Richardson, D. M.: Scientists' warning on invasive alien species, Biol. Rev., 95, 1511–1534, https://doi.org/10.1111/brv.12627, 2020.
R Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 15 November 2020), 2022.
Reid, J. L. and Holl, K. D.: Arrival ≠ survival. Restor. Ecol., 21,153-155, https://doi.org/10.1111/j.1526-100X.2012.00922.x, 2013.
Reis, A., Bechara, F. C., Tres, D. R., and Trentin, B. E.: Nucleação: concepção biocêntrica para a restauração ecológica, Cienc. Florest., 24, 509–519, https://doi.org/10.5902/1980509814591, 2014.
Sant'anna, C. S., Tres, D. R., and Reis, A.: Restauração ecológica: sistemas de nucleação, São Paulo, Secretaria do Meio Ambiente do Estado de São Paulo, ISBN 978-85-86624-73-5, 2011.
SEMA – Secretaria Do Meio Ambiente: Portaria Sema no. 79, de 31 de outubro de 2013, Reconhece a Lista de Espécies Exóticas Invasoras do Estado do Rio Grande do Sul e demais classificações, estabelece normas de controle e dá outras providências, http://www.sema.rs.gov.br/upload/arquivos/201810/02143817-23180118-portaria-sema-79-de-2013-especies (last access: 20 August 2020), 2013.
Sick, H.: Ornitologia Brasileira, Nova Fronteira, Rio de Janeiro, ISBN 978-8520908167, 1997.
Timm, C. D. and Timm, V. F.: Aves do extremo sul do Brasil: guia de identificação, Editora USEB, Rio Grande do Sul, ISBN 978-85-89985-32-1, 2016.
Tomazi, A. L., Zimmermann, C. E., and Laps, R. R.: Poleiros artificiais como modelo de nucleação para restauração de ambientes ciliares: caracterização da chuva de sementes e regeneração natural, Biotemas, 23, 125–135, https://doi.org/10.5007/2175-7925.2010v23n3p125, 2010.
Tubelis, D. P.: Fruit consumption by Colaptes campestris (Aves, Picidae) at Emas National Park, Brazil, Biotemas, 20, 131–133, 2007.
Van der Pijl, L.: Principles of dispersal in higher plants, Springer-Verlag, Berlim, ISBN 978-3-642-87925-8, 1982.
Vissoto, M., Vizentin-Bugoni, J., Bonnet, O. J., Gomes, G. C., and Dias, R. A.: Avian frugivory rates at an abundant tree species are constant throughout the day and slightly influenced by weather conditions, J. Ornithol., 160, 655–663, https://doi.org/10.1007/s10336-019-01663-w, 2019.
Ziller, S. R.: Os processos de degradação ambiental originados por plantas exóticas invasoras, Ciência Hoje, 30, 77–79, 2001.
Zuur, A., Ieno, E., Walker, N., Saveliev, A. and Smith, G.: Mixed Effects Models and extensions in ecology with R, series: Statistics for Biology and Health, Springer, https://doi.org/10.1007/978-0-387-87458-6, 2009.
Zwiener, V. P., Cardoso, F. C., Padial, A. A., and Marques, M. C.: Disentangling the effects of facilitation on restoration of the Atlantic Forest, Basic Appl. Ecol., 15, 34–41, https://doi.org/10.1016/j.baae.2013.11.005, 2014.