the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Experimental warming and permafrost thaw decrease soil nematode abundance in northern palsa peatlands
Hanna Lee
Inge Althuizen
Martijn L. Vandegehuchte
Global warming is profoundly impacting northern ecosystems, particularly those underlain by permafrost. Permafrost-affected peat plateaus, called palsa peatlands, consist of mounds with peat and mineral soil covering ice lenses. When permafrost thaws, palsas can collapse and undergo significant hydrological changes to form wet mires. This affects the physical structure of the soil and as a result, the communities of soil-dwelling organisms, such as nematodes. Although the role of nematodes in carbon cycling is not fully understood, they can influence greenhouse gas emissions through interactions with plants and microbes. This study examined the effects of palsa degradation and experimental warming on nematode feeding guilds (bacterivores, fungivores, root feeders, and omni-carnivores) in northern Norway, where permafrost is rapidly thawing. Our findings showed that intact, vegetated palsas supported higher abundances of all nematode feeding guilds. With warming, bacterivorous and omni-carnivorous nematodes were negatively affected. Additionally, we observed a shift in dominance of bacterivores to fungivores over the summer, suggesting a temporal shift in the predominant decomposition pathway. No direct relationships were found between changes in any of the guild abundances and measured CO2 and CH4 fluxes. This study highlights the fact that expected warming and the degradation of palsas may have varied but had predominantly negative impacts on different nematode feeding guilds. Given the role of soil nematodes in nutrient cycling and other soil processes, their decline under warmer conditions could have ecosystem-level consequences in these palsa peatlands.
- Article
(3553 KB) - Full-text XML
-
Supplement
(410 KB) - BibTeX
- EndNote
1.1 Palsa collapse and climate change
Global-scale warming is accelerating the thawing of permafrost soils, which encompass about 15 % of the Northern Hemisphere's land surface (Obu, 2021) and contain roughly 1300 (± 200) petagrams (1 Pg = 1015 g) of soil organic carbon (Hugelius et al., 2020). Northern peatlands store 415 ± 150 Pg of carbon (C) and 10 ± 7 Pg of nitrogen (N), with nearly half of this area underlain by permafrost (Hugelius et al., 2020). Arctic surface temperatures have risen by around 0.6 °C per decade over the last 40 years, a rate 3 to 4 times faster than the global average soil temperature increase (Rantanen et al., 2022; Li et al., 2023). This has made arctic permafrost areas the fastest warming land on Earth during the last 20 years (Wang et al., 2022). Permafrost thawing exposes previously frozen organic matter to accelerated microbial decomposition, which can release greenhouse gases (GHGs) such as CO2 and CH4 back into the atmosphere (Knoblauch et al., 2018; Schuur and Abbott 2011), exacerbating global warming through a positive feedback loop (Schädel et al., 2024; Schuur et al., 2022). This permafrost–carbon feedback is expected to augment global warming by 0.13–0.27 °C by 2100 and by up to 0.42 °C by 2300 (Schuur et al., 2015).
Permafrost-affected peat plateaus, called palsas, are characterized by peat-covered hills or mounds with a permanently frozen core containing large ice lenses (Seppälä, 2011). Palsas undergo a cyclic formation–degradation process (Fronzek et al., 2006). Their formation relies on prolonged sub-zero temperatures and thin snow cover (Fronzek et al., 2009; Seppälä, 2011). As they mature, degradation occurs, where they start to collapse from the edges due to block erosion along surface cracks, leading to the loss of their insulating peat layer and the thawing of their frozen core, ultimately transforming the landscape into a palsa–mire complex containing ponds or thermokarst (Fronzek et al., 2006). Climate warming, however, threatens this cycle, with an increased frequency of palsa collapse due to accelerated permafrost thaw linked to rising temperatures and rainfall amounts (Borge et al., 2017; Olvmo et al., 2020). These climatic shifts could lead to a substantial reduction in areas suitable for palsa development, with some models predicting the total disappearance of such regions in sub-arctic Fennoscandia by the end of the 21st century (Fronzek et al., 2006; Fewster et al., 2022). Permafrost thaw in palsa peatlands results in waterlogged conditions favourable for increased methane production (Hodgkins et al., 2014; Singleton et al., 2018; Yu et al., 2017). Understanding and quantifying the link between permafrost thaw and greenhouse gas emissions is therefore important to improve predictions of future global warming (Hodgkins et al., 2014; Singleton et al., 2018; Yu et al., 2017).
Recent research highlights the importance of soil food webs in regulating greenhouse gas emissions during organic matter decomposition, particularly in processes such as methanogenesis and soil respiration (Mackelprang et al., 2016; McCalley et al., 2014; Singh et al., 2010; Voigt et al., 2023). Soil nematodes, as key components of these webs, can serve as useful indicators of microbial activity and the balance between bacteria and fungi in soils (Cesarz et al., 2015; Yeates, 2003). Furthermore, their interactions with microbes and vegetation can influence CO2 and CH4 emissions. Therefore, studying nematodes can yield insights into the ecological shifts driving greenhouse gas dynamics in response to environmental changes.
1.2 The role of nematodes in the carbon cycle and their main abiotic drivers in the Arctic
Nematodes, or roundworms, are a diverse phylum of aquatic microfauna (0.1–5 mm in length) that can live in water films around soil particles (Neher, 2010). They have diverse feeding habits, which could ultimately affect the biological activity involved in soil organic matter decomposition. Soil nematodes can be categorized into different feeding guilds, with five typically recognized groups: (a) bacterial-feeding nematodes or bacterivores, (b) fungal-feeding nematodes or fungivores, (c) root-feeding nematodes or root feeders, (d) omnivores, and (e) predators (Bongers and Bongers, 1998). These guilds hold a central position in soil food webs and play crucial roles in the cycling of soil carbon and nutrients (Bardgett et al., 1999). For example, bacterivores and predatory nematodes contribute to nitrogen mineralization by excreting ammonium as a byproduct because their prey generally has a lower carbon to nitrogen ratio than these nematodes need (Neher, 2010). Microbivorous nematodes (i.e. bacterivores and fungivores) can increase the microbial turnover (i.e. the rate of cell production and death) of bacteria and fungi, enhancing overall soil productivity and nutrient cycling (Savin et al., 2001; Traunspurger et al., 1997), and, potentially, CO2 production (Fu et al., 2005). Finally, while root-feeding nematodes include some notorious plant pests, they may also indirectly boost plant performance by promoting microbial populations in the rhizosphere by stimulating plant exudate release (Gebremikael et al., 2016; Poll et al., 2007; Tu et al., 2003; Wurst et al., 2010). Each nematode feeding guild contributes to CO2 emissions through aerobic respiration (Atkinson, 1980) and could influence the net GHG emissions by affecting other soil fauna, soil microbes, or vegetation. Bacterivores and omnivores may either restrict or stimulate CH4 emissions depending on their grazing intensity on methanotrophic bacteria and methanogenic archaea populations. Root-feeding nematodes could decrease photosynthesis (CO2 removal) by affecting plant activity or stimulate CO2 production in the rhizosphere by promoting root leakage of labile carbon sources for microbial growth and respiration (Gebremikael et al., 2016). Lastly, predatory nematodes may indirectly alter emissions by regulating other feeding guilds, thereby influencing microbial dynamics, vegetation, and nutrient cycling (Neher, 2010).
Nematode distribution globally is known to be primarily influenced by soil properties such as texture, temperature, organic matter content, pH, and cation-exchange capacity (Nielsen et al., 2014; van den Hoogen et al., 2019). However, climate factors, particularly rainfall, play a significant role by altering soil characteristics, contributing as much as 65 % of the global variation in nematode populations (Nielsen et al., 2014). In cold environments, freeze–thaw cycling frequency can negatively affect the abundance of nematodes (Knox et al., 2016), and changes associated with permafrost thaw such as altered soil texture, pH, and salinity may also affect nematode distribution (Smith et al., 2012). Globally, nematode abundance peaks in high-latitude arctic and sub-arctic regions (van den Hoogen et al., 2019). Therefore, the relative importance of nematodes and their role in cold regions, particularly those undergoing rapid changes due to permafrost thawing, warrant closer investigation to increase the predictability of greenhouse gas emissions in this region.
1.3 Hypotheses
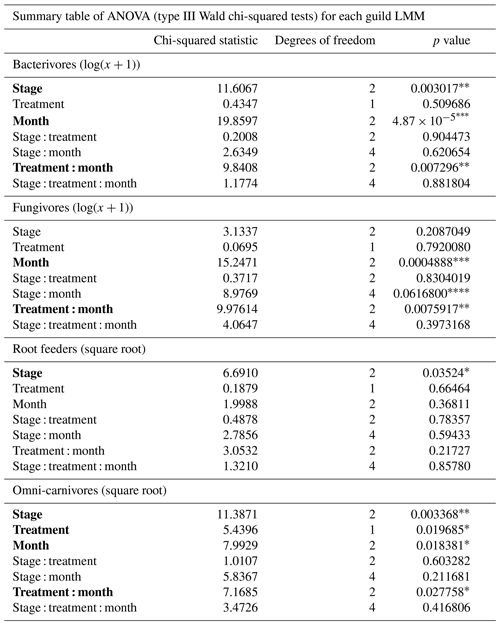
This study investigated the variations in nematode feeding guild abundances across different stages of palsa degradation in a palsa peatland system undergoing rapid permafrost degradation in northern Norway. We also explored the effects of experimental warming along different stages of palsa degradation on the nematode communities. We hypothesize that palsa collapse through increased soil temperature and moisture, along with decreased oxygen concentration (Tromans, 2000; Walvoord and Kurylyk, 2016), will have varying effects on the different guilds. Specifically, we expect microbivorous nematodes (opportunistic feeders) to benefit from palsa collapse due to increased microbial activity, while root-feeding nematodes will decrease in number due to diminished host plant availability. Additionally, we hypothesize that experimental warming will positively affect nematodes by improving microenvironments and resource utilization in soil habitats (Liu et al., 2022) or by stimulating vegetation growth (Hollister et al., 2023; Newsham et al., 2021). However, we predict a negative impact of both collapse and warming on omnivorous and predatory nematodes (together the omni-carnivores) because these guilds are sensitive to physical disturbance due to their low motility, slow life cycle, and permeable cuticle (Bongers and Bongers, 1998; Korthals et al., 1996; Pothula et al., 2022). Finally, shifts in nematode guild abundances are anticipated to influence GHG emissions, as they play pivotal roles in microbial activities and/or plant–microbe interactions. We used structural equation models (SEMs) to integrate these various hypothesized causal pathways into a single, multivariate analysis (for details, see Table 1).
2.1 Field site, experimental design, and peat characteristics
We conducted our research at a permafrost-affected palsa peatland in northern Norway (69.3° N, 25.3° E, 337 m a.s.l. (metres above sea level)) close to the hill called Iškoras (Fig. 1a; Jiao et al., 2023). The site is characterized by a mean seasonal air temperature of 8 to 10 °C in summer and −15 to −20 °C in winter (MAT ca. −3 °C) (retrieved from the Norwegian Meteorological Institute). The plateau has a mean annual precipitation of 400 mm (Borge et al., 2017) and a mean maximum snow depth in winter of 25 to 75 cm (Farbrot et al., 2013). The site represents natural gradients of permafrost thaw, from palsa peat plateaus with intact permafrost through thaw slumps to mire-like thaw ponds as thaw progresses. Eventually, these thaw ponds are (re)colonized, developing vegetation through natural succession with mosses and sedges. At the site, six such transects of palsa collapse were selected, each containing five phases of permafrost degradation: vegetated palsa, exposed soil palsa, thaw slump, thaw pond, and vegetated thaw pond (Fig. 1b). Soil samples were collected in the first three phases, and water samples were collected in the pond phases as described below (Sect. 2.2). However, due to a lack of abundance of soil nematodes found in the water samples, we chose to exclude the two pond phases from this study. Additionally, in one transect, no soil samples were collected from the thaw slump phase because it was fully submerged. Every palsa collapse stage within a transect has a control plot and a treatment plot to study experimental warming performed by open-top chambers (OTCs). An OTC is a warming chamber that passively warms the air temperature by approximately 2 °C, with the size of its warming effect on soil temperatures varying due to factors like location, shading, and ground cover (Hollister et al., 2023). These OTCs, 150 cm in diameter at the base and 35 cm tall, were established in 2017 and remain in place throughout all seasons.
Soil temperature was measured manually using a thermometer during sampling, as well as logged continuously at 15 min intervals with TMS-4 loggers (TOMST; Wild et al., 2019). The average temperature, minimum temperature, and maximum temperature of the week prior to and the week of sampling were calculated from these continuous measurements. To estimate soil moisture content, 50 g from each 100 g soil sample (see below) was oven-dried at 105 °C for 24 h, while oxygen levels were measured in situ for 2 min while soil samples were being collected using a Pyroscience FireSting-O2 fibre optic oxygen meter.
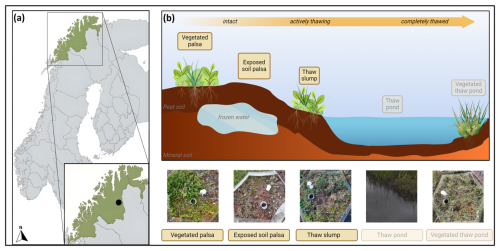
Figure 1(a) Location of the research site; (b) schematic overview of the five landscape types along each thaw gradient (n=6). Shaded landscape types were not included due to the low presence of nematodes. Map created with https://mapchart.net (last access: 18 January 2024). Figure created with BioRender.
2.2 Soil sampling and nematode extraction
During the first week of June, July, and August 2022, soil samples were collected from each vegetated palsa, exposed soil palsa, and thaw slump plot. In each plot, three soil sub-samples (10 cm depth, 2 cm diameter) were combined into a single 100 g sample. This was done for both control and OTC-treated plots across all six transects, resulting in a total of 108 soil samples (6 transects × 3 thaw stages × 2 treatments × 3 months). Samples were stored in a refrigerator during fieldwork and transported back to the laboratory in a cooler with ice packs to slow down nematode metabolism. Thaw pond phases were sampled by collecting 5 cl water in three locations. In the lab, nematodes were extracted from the remaining 50 g soil using Baermann funnels (Cesarz et al., 2019; Tintori et al., 2022). These glass funnels contained a flat, circular cup of metal mesh lined with tissue paper in which the soil sample was placed. Nematodes and other microfauna moving through the tissue paper are collected in the stem of the funnel, which has a piece of rubber tubing with a metal clamp. Every 24 h, for 3 consecutive days, 10 mL was drawn from the funnels. From each 30 mL sample, after a 24 h settling period in the refrigerator for the nematodes to settle, the top 25 mL was removed using vacuum suction, leaving a concentrated 5 mL sample. This was mixed with 5 mL of 5 % hot formaldehyde (60–70 °C) to fix and preserve the animals.
The nematodes were classified under an inverted microscope (at 200× and 400× magnification) into the five main feeding guilds based on their morphological traits. These were the morphology of the feeding apparatus, bulbs of the pharynx, and size. In some cases, nematodes were classified as “unknown” due to difficulties in identification caused by their (partial) decomposition prior to fixation (on average 6 % of nematodes per sample). However, since the predator guild had too few individuals for meaningful analysis as a separate feeding guild, we combined omnivores and predators into a single omni-carnivorous group, as these guilds are ecologically similar (Yeates et al., 1993). The count data for each feeding guild were normalized to nematodes per gram of dry soil based on the gravimetric soil moisture determined earlier.
2.3 Soil respiration measurements
We conducted soil respiration measurements (CO2 and CH4) in the same weeks as the soil sampling. Soil respiration was measured on 20 cm long cylindrical PVC soil collars (11 cm in diameter) that were inserted 10 cm into the ground in 2017 at each plot. For each measurement, the collar headspace was sealed with a tight-fitting PVC lid (DBI-dut Nr121B) connected to an infrared-gas analyser (Li7810, LI-COR Biosciences, Lincoln, NE, USA) for a period of 80–120 s. The height of the collar was measured at four points for each measurement in order to calculate accurate headspace volumes based on mean collar height, as soil subsidence has affected the placement of soil collars over time. Soil respiration rates (CO2 and CH4) were calculated using the HMR package (Pedersen et al., 2010), selecting either linear or non-linear regression where appropriate and converting from µL m−2 s−1 to µmol m−2 s−1 using the standard gas constant adjusted for temperature during the measurement based on plot-level TOMST logger data.
2.4 Statistical analyses
All data analyses were conducted using R v4.2.2. (R Core Team, 2013). Spearman's rank correlations between the measured soil conditions were calculated using the base cor() function (Fig. S1). Linear mixed models (LMMs) were created to study changes in the abundance of the individual feeding guilds (bacterivores, fungivores, root feeders, and omni-carnivores). These LMMs included the palsa degradation phase (stage), warming treatment (OTC), time (month), and their interactions as categorical fixed effects. Three levels of random effects were specified: “transect” adjusts for common traits among samples from the same transect, “stage within transect” accounts for relationships among samples at the same degradation stage within a transect, and “treatment within stage within transect” considers dependencies among samples from the same plot across the 3 months. Assumptions of residual normality and homoscedasticity were tested using the shapiro.test() function from the stats package (R Core Team, 2013) and the leveneTest() function from the car package, respectively (Fox and Weisberg, 2019). Transformations (log(x+1) or cube root) were applied to every model except the temperature and moisture to accommodate violations of these assumptions. Significance of fixed effects was tested with a type III Wald chi-squared test using the Anova() function from the rstatix package (Kassambara, 2023). Post hoc analyses were conducted using the emmeans() function from the emmeans package (Lenth, 2024) using the false discovery rate (FDR) to correct for multiple comparisons, and the significant difference was summarized using a compact letter display (CLD) using the cld() function from multcomp (Hothorn et al., 2008). Similar models were created to test changes in peat characteristics (soil temperature, moisture, oxygen concentration, and GHG fluxes), which can be found in the appendix (Table S1).
Structural equation models (SEMs) were created to test multivariate hypotheses about the mechanistic drivers behind the observed patterns in the linear mixed models. SEMs are probabilistic models that unite multiple predictor and response variables into a single causal network, representing a broad range of multivariate hypotheses about interdependencies (Lefcheck, 2016). A base model was defined a priori to predict hypothesized relationships among changes in nematode guild abundances, soil conditions, gas fluxes, and the three predictors from the LMMs: palsa stage, warming treatment, and month (Fig. 2) (Table 1). We decided to only include the thermometer measurements due to their strong correlation with the temperature metrics calculated from the TOMST readings (Fig. S1). Shipley's tests of directed separation (Lefcheck, 2016) were used to test the independence claims, which involve checking if any unspecified arrows could be included in the model structure to increase its fit. However, adding a path or arrow should only be done if it is theoretically and ecologically justifiable, as adding arrows through iteratively refining an SEM amounts to model dredging. Initially, SEMs were developed, and subsequently tested, on each feeding guild separately using the psem() function from the piecewiseSEM package (Lefcheck, 2016). For this, a linear mixed model (with the same random effects as before) was specified for each endogenous variable in each SEM. During this process, all relevant assumptions were checked, and appropriate transformations were applied when necessary. More specifically, bacterivore and fungivore abundance were log(x+1) transformed, while root feeder and omni-carnivore abundance, as well as CO2 and CH4 fluxes, were cube root transformed. The only appropriate model for soil moisture was a generalized linear mixed model with beta regression, but the psem() function is not compatible with these kind of models. Therefore, we opted for a basic linear mixed model on the square root of moisture, of which the residuals slightly violated the assumption of normality. Finally, one large SEM was developed including all feeding guilds.
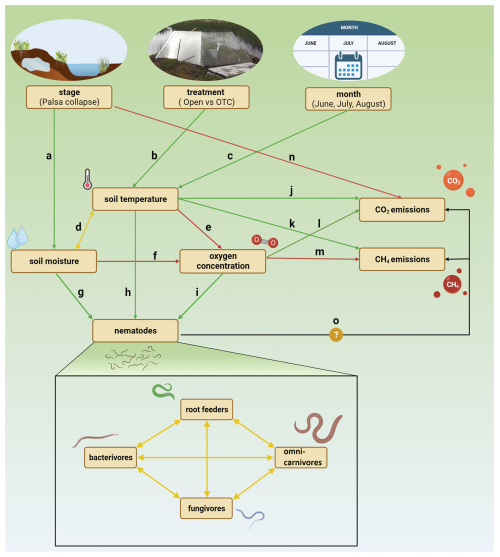
Figure 2SEM base model, with hypothesized positive relationships represented by green and negative by red directional arrows, and bidirectional relationships by yellow bidirectional arrows. For the sake of visual clarity the variable “nematodes” was added. In reality, the arrows “g”,” h”, “i”, and “o” are connected to each guild. Created with BioRender.
3.1 Effect of palsa collapse, experimental warming, and seasonal progression on feeding guild abundances
A total of 17 488 nematodes were identified to the feeding guild level. The linear mixed models revealed significant effects of palsa collapse, warming treatment, and/or month for each guild (Table 2), as well as for total nematode abundance (Fig. S2; Table S2). Intact, vegetated palsas consistently showed higher abundances of all nematode feeding guilds, although the difference was not significant for fungivores (Fig. 3). Specifically, bacterivore abundance was significantly higher in intact, vegetated palsas compared to both exposed palsas and thaw slumps. Bacterivore abundance peaked in July, with significantly lower abundances in August compared to June and July. A significant interaction between warming treatment and month was observed for bacterivores, with warming decreasing bacterivore numbers in June and August but not in July.
The abundance of fungivores did not differ significantly among the palsa stages. However, fungivore abundance increased significantly as the summer progressed, with higher abundances in July and August compared to June. A significant interaction between warming treatment and month was also found for fungivores, with no effect of warming on fungivore numbers in June, a small increase with warming in July, and a stronger decrease in August. Root feeders showed a significantly higher abundance in intact, vegetated palsas compared to exposed palsas and thaw slumps. No additional significant effects were found for this feeding guild.
Omni-carnivores were also significantly more abundant in intact, vegetated palsas compared to exposed palsas and thaw slumps. A significant interaction between warming treatment and month was also observed for this guild, with a decrease in omni-carnivore numbers in warmed plots in June and August but no warming effect in July.
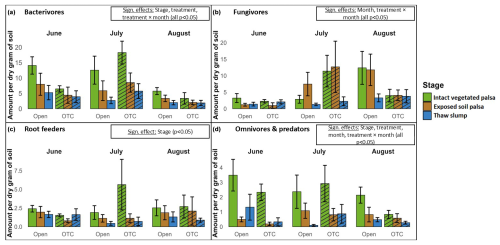
Figure 3Nematode feeding guild abundances across palsa degradation stages, warming treatments, and months. The y axis shows non-transformed counts of each group per gram of dry soil. Diagonal striping indicates treatment (OTC).
3.2 Multivariate relationships among palsa collapse, warming treatment, peat characteristics, and guild abundances
The structural equation models (SEMs) for each feeding guild achieved an adequate fit after including additional relationships based on tests of directed separation. Thaw stage, warming treatment, and seasonal progression emerged as primary drivers of peat conditions, with thaw stage exerting a particularly strong influence. For bacterivores, the SEM included a direct effect of month on abundance, indicating that seasonal changes not captured by measured variables influenced bacterivore abundance. A negative relationship was observed between soil temperature and bacterivore abundance. The fungivore SEM also included a direct effect of month on abundance, reflecting the significant increase in fungivore abundance over the summer that is due to more than the changes in the soil characteristics included over the summer.
In the root feeder SEM, a direct effect of thaw stage on abundance was included, suggesting that root feeder abundance is directly influenced by the stage of palsa collapse. For omni-carnivores, the SEM also included a direct effect of the stage of palsa collapse but also of warming treatment on their abundance, as well as a significant, negative relationship between omni-carnivore abundance and soil temperature. This indicates that both palsa collapse and warming treatments directly affect omni-carnivore populations through more than just changes in soil temperature.
The combined SEM, encompassing all feeding guilds and CO2 and CH4 emissions, showed no significant relationships between the abundances of any feeding guild and the fluxes of CO2 and CH4 (Fig. 4). The model revealed the same significant relationships between thaw stage and soil conditions and between soil conditions and nematode guild abundances as the individual guild models. To obtain adequate model fits, the direct effects of palsa stage on soil temperature, soil moisture, and oxygen concentration were added. Additionally, positive covariances were observed among the abundances of all nematode feeding guilds, suggesting that the abundances of different guilds are positively correlated.
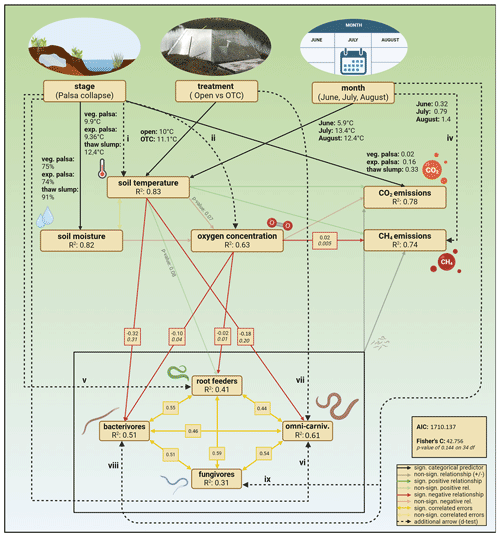
Figure 4Final SEM, showing the significant and non-significant hypothesized relationships from the base model. Dashed arrows are additional arrows added to increase model fit based on d-separation tests. Back-transformed estimated marginal means of soil moisture, soil temperature, and CO2 emissions for each stage, treatment, and month were added next to their respective arrows. Adjusted R2 values for each endogenous variable indicate the variance explained by the model. Significant path coefficients are presented in raw (normal) and standardized (italic) units. Created with https://BioRender.com (last access: 17 March 2025).
4.1 All nematode guilds thrive in intact, vegetated palsas
The different nematode feeding guilds were most abundant in the intact vegetated palsas, suggesting that future palsa collapse could negatively impact nematodes due to changes in soil conditions or shifts in vegetation composition. This result contradicts our hypothesis partly, as we anticipated an increase in bacterivore and fungivore abundance under palsa degradation. However, the higher abundance of root feeders and omni-carnivores in intact, vegetated palsas supports our hypothesis and the existing literature and acts as a reminder that the presence of omnivores and predators can indicate a diverse, stable soil food web, while their absence may signal soil disruption or nutrient depletion (Bongers and Bongers, 1998; Pothula et al., 2022).
While previous studies have shown that vegetation positively influences nematodes by enhancing microbial decomposition and directly supporting root feeders (Gebremikael et al., 2016; Wu et al., 2021; Yurkevich et al., 2020), this relationship alone does not fully explain our findings. For example, thaw slumps, despite being vegetated, exhibited significantly lower nematode abundances similar to those observed in exposed soil palsas. This discrepancy underscores the importance of additional factors beyond vegetation presence. Intact palsas likely provide a more stable microenvironment with well-drained and aerated soil conditions, which promote diverse microbial communities and favourable habitats for nematodes (De Deyn et al., 2004; Maharning et al., 2016; Wu et al., 2021). In contrast, thaw slumps are characterized by wetter soils and reduced oxygen levels, which might suppress nematode populations despite vegetation cover (Grant and Villani, 2003; Neher, 2010). Moreover, differences in vegetation composition between intact palsas and thaw slumps may further influence nematode communities, as plant species can shape the availability of resources and microhabitats (Verschoor et al., 2002; Viketoft et al., 2005; Yurkevich et al., 2020).
4.2 Negative effects of increasing soil temperature on bacterivores and omni-carnivores
Contrary to our hypothesis, we found a negative effect of increasing soil temperature on bacterivore and omni-carnivore abundance. The SEMs revealed a negative relationship between soil temperature and bacterivore abundance, suggesting that rising temperatures along thaw gradients, from seasonal progression, and in our warming treatments contributed to the observed declines. For example, the early summer peak in bacterivore populations could partly be attributed to lower average soil temperatures in June (5.9 °C) compared to July and August (12.4 and 13.4 °C, respectively).
Similar observations were reported by Simmons et al. (2009), who found a negative impact of soil warming on bacterivores in the McMurdo Dry Valleys, Antarctica – likely due to increased moisture suppressing a dominant bacterivore species adapted to dry conditions. In contrast, Ruess et al. (1999) reported higher nematode densities in warm, wet Arctic soils, indicating a positive temperature effect. These results suggest that the impact of warming on nematodes may be influenced by interactions with other environmental factors such as moisture. The negative correlation between soil temperature and both bacterivore and omni-carnivore abundance in our study suggests that future global warming could lead to declines in these guilds and their associated ecosystem functions in palsa peatlands.
Although our measurements confirmed that soil moisture increased and oxygen concentration declined with palsa collapse, neither variable emerged as a strong driver of nematode feeding guilds in our models. Only a small negative path coefficient was detected from oxygen to bacterivores and root feeders, likely reflecting the broad physiological tolerance of nematodes to mildly hypoxic conditions (Van Voorhies and Ward, 2000) and micro-scale variations in soil aeration. Moreover, soil moisture in our study site remained consistently high (62 %–95 %), a range presumably comfortable for nematodes across all feeding guilds.
4.3 Bacterivores and fungivores show opposite temporal dynamics
The decrease in bacterivore abundance and simultaneous increase in fungivore abundance over the summer could be driven by a summer shift from bacteria- to fungi-dominated decomposition. We hypothesize that initially, as the active layer thickens, opportunistic bacteria rapidly consume labile compounds, followed by a peak and then a decline in bacterivores as these compounds become scarce (Mueller et al., 2015). The persistence of some bacterivores in August may be due to increased vegetation development stimulating bacterial activity through exudate release (Gebremikael et al., 2014), while the abundance of fungi in late summer could also support bacterial populations, as fungi usually initiate plant material decomposition (Neher, 2010).
Unlike bacterivore abundance, which decreased by the end of the summer, fungivores significantly increased from June to August. We propose three hypotheses that may explain this trend. Firstly, rising summer temperatures could lead to an accumulation of recalcitrant plant litter only decomposable by fungi – the primary food for fungivorous nematodes (Biasi et al., 2005; Otten et al., 2001; Xu et al., 2015). Secondly, increased vegetation density over the summer could directly benefit fungivores through root exudates stimulating fungi in the rhizosphere (Gebremikael et al., 2014; Gebremikael et al., 2016). Lastly, the time required for fungi to develop their hyphal network after seasonal soil thaw might lead to a delayed increase in fungivore abundance, which could explain the low levels observed in early summer, when not enough mild days had passed for extensive fungal growth (Gostinčar et al., 2022).
4.4 All guilds positively covary
The SEM including all guilds highlights a significant positive covariance among all feeding guild abundances, even when accounting for direct and indirect relationships with thaw stage, warming treatment, month, and measured peat conditions. Due to the limited existing literature on specific interactions between nematode guilds, we chose not to incorporate directional arrows among the feeding guilds in the SEM. Community assembly theory suggests that non-random species assembly results from mechanisms like environmental filtering and/or biotic interactions, namely facilitative (indirect) interactions (Sutton et al., 2021; Van De Walle et al., 2023). Plant-feeding nematodes, for example, can facilitate the release of exudates into the soil, stimulating microbial growth and decomposition, which could benefit microbivorous nematode populations (Gebremikael et al., 2016; Poll et al., 2007; Tu et al., 2003; Wurst et al., 2010). Additionally, bacterivores, fungivores, and omnivores feed on microbes and excrete excess nutrients into the soil (Neher, 2010), enhancing plant productivity and benefitting all feeding guilds. Microbivorous nematodes could also stimulate microbial growth by transporting microbes to nutrient-rich microenvironments (Fu et al., 2005) or by accelerating microbial population turnover (Savin et al., 2001; Traunspurger et al., 1997). These mechanisms promote microbial growth and nutrient availability, ultimately benefiting the entire nematode community (Bardgett et al., 1999; Gebremikael et al., 2014).
4.5 Broader implications and future prospects
Our findings did not reveal strong connections between the abundances of different nematode feeding guilds and the fluxes of greenhouse gases, suggesting that the role of nematodes may not be as substantial as other drivers such as environmental drivers. Consequently, we did not observe strong relationships between the observed shift from bacterial-to-fungal dominance among microbivorous nematodes over the summer and GHG emissions. This shift in the community structure of microbivorous nematodes, however, reflects a change in the primary decomposition pathway, indicating alterations in organic matter dynamics and microbial activity. As nematodes play critical roles in nutrient cycling (Bardgett et al., 1999; Savin et al., 2001) and influence plant growth dynamics (Gebremikael et al., 2016; Neher, 2010), changes in their community composition could indirectly affect greenhouse gas emissions over longer timescales or under different environmental conditions. The intricate relationships within the soil food web, including those involving nematode feeding guilds, need further exploration to better understand their potential impacts on greenhouse gas emissions, particularly under the rapidly changing conditions of palsa peatlands. The positive covariance between all feeding guilds, for example, could mean that changes in one guild will have cascading effects on others. Therefore, understanding these relationships is crucial, as they could reveal broader ecological impacts, such as altered nutrient cycles and energy flows, in the context of climate-induced permafrost thaw.
In summary, this study highlights the sensitivity of nematode guilds to palsa collapse and experimental warming, which, coupled with their pivotal role in soil nutrient cycling and other soil processes, positions nematodes as potential indicators of ecosystem health and stability in the face of climate change. Increased soil temperatures, in particular, could directly exacerbate the decline in bacterivores and omni-carnivores. In conclusion, palsa collapse driven by global warming is likely to adversely impact all nematode guilds and the varied functions and services they provide in the ecosystem.
The datasets generated during this study and the R scripts supporting the findings of this study are available on Zenodo at https://doi.org/10.5281/zenodo.15005319 (Van Daele, 2025).
The supplement related to this article is available online at https://doi.org/10.5194/we-25-121-2025-supplement.
HL and IA selected the field transects and designed the warming experiment. MLV designed the nematode sampling campaign. RVD and MLV collected the samples and measured the soil properties. RVD extracted and identified the nematodes and conducted the subsequent data analyses. RVD wrote the paper with contributions from all authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors would like to thank Omar Orozco Granados for his invaluable assistance in identifying nematodes and contributing to the sampling of experiments in June and thank Lisa van Solt and Anja Greschkowiak for conducting gas measurements. Gratitude is also extended to the Norwegian University of Science and Technology (NTNU) for providing access to facilities and resources that were instrumental in conducting this research.
This research has been supported by the Research Council of Norway Project (grant no. 309625 for Hanna Lee).
This paper was edited by Erinne Stirling and reviewed by Sara Sánchez Moreno and one anonymous referee.
Atkinson, H. J.: 5 – Respiration in nematodes, in: Aging and Other Model Systems, edited by: Zuckerman, B. M., Acad. Press, 101–142, https://doi.org/10.1016/B978-0-12-782402-4.50011-9, 1980.
Bakonyi, G., Nagy, P., Kovács-Láng, E., Kovács, S., Barabás, S., Répási, V., and Seres, A.: Soil nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland, Appl. Soil Ecol., 37, 31–40, https://doi.org/10.1016/j.apsoil.2007.03.008, 2007.
Bardgett, R. D., Cook, R., Yeates, G. W., and Denton, C. S.: The influence of nematodes on below-ground processes in grassland ecosystems, Plant Soil, 212, 23–33, https://doi.org/10.1023/A:1004642218792, 1999.
Biasi, C., Rusalimova, O., Meyer, H., Kaiser, C., Wanek, W., Barsukov, P., Junger, H., and Richter, A.: Temperature-dependent shift from labile to recalcitrant carbon sources of Arctic heterotrophs, Rapid Commun. Mass Spectrom., 19, 1401–1408, https://doi.org/10.1002/rcm.1911, 2005.
Bongers, T. and Bongers, M.: Functional diversity of nematodes, Appl. Soil Ecol., 10, 239–251, https://doi.org/10.1016/S0929-1393(98)00123-1, 1998.
Borge, A. F., Westermann, S., Solheim, I., and Etzelmüller, B.: Strong degradation of palsas and peat plateaus in northern Norway during the last 60 years, The Cryosphere, 11, 1–16, https://doi.org/10.5194/tc-11-1-2017, 2017.
Cesarz, S., Reich, P. B., Scheu, S., Ruess, L., Schaefer, M., and Eisenhauer, N.: Nematode functional guilds, not trophic groups, reflect shifts in soil food webs and processes in response to interacting global change factors, Pedobiologia, 58, 23–32, https://doi.org/10.1016/j.pedobi.2015.01.001, 2015.
Cesarz, S., Schulz, A. E., Beugnon, R., and Eisenhauer, N.: Testing soil nematode extraction efficiency using different variations of the Baermann-Funnel method, Soil Organ., 91, 61–72, https://doi.org/10.25674/so91201, 2019.
Chen, X., Xue, D., Wang, Y., Qiu, Q., Wu, L., Wang, M., Liu, J., and Chen, H.: Variations in the archaeal community and associated methanogenesis in peat profiles of three typical peatland types in China, Environ. Microbiom., 18, 48, https://doi.org/10.1186/s40793-023-00503-y, 2023.
De Deyn, G. B., Raaijmakers, C. E., Van Ruijven, J., Berendse, F., and Van Der Putten, W. H.: Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web, Oikos, 106, 576–586, https://doi.org/10.1111/j.0030-1299.2004.13265.x, 2004.
Dong, Z., Hou, R., Chen, Q., Ouyang, Z., and Ge, F.: Response of soil nematodes to elevated temperature in conventional and no-tillage cropland systems, Plant Soil, 373, 907–918, https://doi.org/10.1007/s11104-013-1846-2, 2013.
Farbrot, H., Isaksen, K., Etzelmüller, B., and Gisnås, K.: Ground thermal regime and permafrost distribution under a changing climate in northern Norway, Permafr. Periglac. Process., 24, 20–38, https://doi.org/10.1002/ppp.1763, 2013.
Feng, G., Wu, L., and Letey, J.: Evaluating aeration criteria by simultaneous measurement of oxygen diffusion rate and soil–water regime, Soil Sci., 167, 495–503, https://doi.org/10.1097/00010694-200208000-00001, 2002.
Fewster, R. E., Morris, P. J., Ivanovic, R. F., Swindles, G. T., Peregon, A. M., and Smith, C. J.: Imminent loss of climate space for permafrost peatlands in Europe and Western Siberia, Nat. Clim. Change, 12, 373–379, https://doi.org/10.1038/s41558-022-01296-7, 2022.
Fox, J. and Weisberg, S.: An R Companion to Applied Regression, 3rd ed., Sage Publ., https://www.john-fox.ca/Companion/ (last access: 20 May 2025), 2019.
Fronzek, S., Luoto, M., and Carter, T.: Potential effect of climate change on the distribution of palsa mires in subarctic Fennoscandia, Clim. Res., 32, 1–12, https://doi.org/10.3354/cr032001, 2006.
Fronzek, S., Johansson, M., Christensen, T. R., Carter, T., Friborg, T., and Luoto, M.: Climate change impacts on sub-Arctic palsa mires and greenhouse-gas feedbacks, in: Proceedings of the PALSALARM Symposium, Abisko, Sweden, 28–30 October 2008, Reports of the Finnish Environment Institute 3/2009, Finnish Environment Institute (SYKE), Helsinki, ISBN 978-952-11-3362-6, 2009.
Fu, S., Ferris, H., Brown, D., and Plant, R.: Does the positive feedback effect of nematodes on the biomass and activity of their bacterial prey vary with nematode species and population size?, Soil Biol. Biochem., 37, 1979–1987, https://doi.org/10.1016/j.soilbio.2005.01.018, 2005.
Gebremikael, M. T., Buchan, D., and De Neve, S.: Quantifying the influences of free-living soil nematodes on soil nitrogen and microbial biomass dynamics in bare and planted microcosms, Soil Biol. Biochem., 70, 131–141, https://doi.org/10.1016/j.soilbio.2013.12.006, 2014.
Gebremikael, M. T., Steel, H., Buchan, D., Bert, W., and De Neve, S.: Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions, Sci. Rep., 6, 32862, https://doi.org/10.1038/srep32862, 2016.
Gostinčar, C., Zalar, P., and Gunde-Cimerman, N.: No need for speed: Slow development of fungi in extreme environments, Fungal Biol. Rev., 39, 1–14, https://doi.org/10.1016/j.fbr.2021.11.002, 2022.
Grant, J. A. and Villani, M. G.: Soil moisture effects on entomopathogenic nematodes, Environ. Entomol., 32, 80–87, https://doi.org/10.1603/0046-225X-32.1.80, 2003.
Hodgkins, S. B., Tfaily, M. M., McCalley, C. K., Logan, T. A., Crill, P. M., Saleska, S. R., Rich, V. I., and Chanton, J. P.: Changes in peat chemistry associated with permafrost thaw increase greenhouse gas production, P. Natl. Acad. Sci. USA, 111, 5819–5824, https://doi.org/10.1073/pnas.1314641111, 2014.
Hollister, R. D., Elphinstone, C., Henry, G. H. R., Bjorkman, A. D., Klanderud, K., Björk, R. G., Björkman, M. P., Bokhorst, S., Carbognani, M., Cooper, E. J., Dorrepaal, E., Elmendorf, S. C., Fetcher, N., Gallois, E. C., Guðmundsson, J., Healey, N. C., Jónsdóttir, I. S., Klarenberg, I. J., Oberbauer, S. F., Macek, P., May, J. L., Mereghetti, A., Molau, U., Petraglia, A., Rinnan, R., Rixen, C., and Wookey, P. A.: A review of open top chamber (OTC) performance across the ITEX network, Arct. Sci., 9, 331–344, https://doi.org/10.1139/as-2022-0030, 2023.
Hothorn, T., Bretz, F., and Westfall, P.: Simultaneous inference in general parametric models, Biometrical J., 50, 346–363, https://doi.org/10.1002/bimj.200810425, 2008.
Howard, D., Agnan, Y., Helmig, D., Yang, Y., and Obrist, D.: Environmental controls on ecosystem-scale cold-season methane and carbon dioxide fluxes in an Arctic tundra ecosystem, Biogeosciences, 17, 4025–4042, https://doi.org/10.5194/bg-17-4025-2020, 2020.
Hugelius, G., Loisel, J., Chadburn, S., Jackson, R. B., Jones, M., MacDonald, G., Marushchak, M., Olefeldt, D., Packalen, M., Siewert, M. B., Treat, C., Turetsky, M., Voigt, C., and Yu, Z.: Large stocks of peatland carbon and nitrogen are vulnerable to permafrost thaw, P. Natl. Acad. Sci. USA, 117, 20438–20446, https://doi.org/10.1073/pnas.1916387117, 2020.
Jiao, Y., Davie-Martin, C. L., Kramshøj, M., Christiansen, C. T., Lee, H., Althuizen, I. H. J., and Rinnan, R.: Volatile organic compound release across a permafrost-affected peatland, Geoderma, 430, 116355, https://doi.org/10.1016/j.geoderma.2023.116355, 2023.
Karlgård, J.: Degrading Palsa Mires in Northern Europe: Changing Vegetation in an Altering Climate and Its Potential Impact on Greenhouse Gas Fluxes, Master's thesis, Lunds Univ. Naturgeogr. Inst., http://lup.lub.lu.se/student-papers/record/1890250 (last access: 20 May 2025), 2008.
Kassambara, A.: rstatix: Pipe-Friendly Framework for Basic Statistical Tests (Version 0.7.2) [R package], CRAN, https://CRAN.R-project.org/package=rstatix (last access: 20 May 2025), 2023.
Kitazume, H., Dayi, M., Tanaka, R., and Kikuchi, T.: Assessment of the behaviour and survival of nematodes under low oxygen concentrations, PLoS One, 13, e0197122, https://doi.org/10.1371/journal.pone.0197122, 2018.
Knoblauch, C., Beer, C., Liebner, S., Grigoriev, M. N., and Pfeiffer, E. M.: Methane production as key to the greenhouse gas budget of thawing permafrost, Nat. Clim. Change, 8, 309–312, https://doi.org/10.1038/s41558-018-0095-z, 2018.
Knoblauch, C., Beer, C., Schuett, A., Sauerland, L., Liebner, S., Steinhof, A., Rethemeyer, J., et al.: Carbon dioxide and methane release following abrupt thaw of Pleistocene permafrost deposits in Arctic Siberia, J. Geophys. Res.-Biogeosci., 126, e2021JG006543, https://doi.org/10.1029/2021JG006543, 2021.
Knox, M. A., Wall, D. H., Virginia, R. A., Vandegehuchte, M. L., San Gil, I., and Adams, B. J.: Impact of diurnal freeze–thaw cycles on the soil nematode Scottnema lindsayae in Taylor Valley, Antarctica, Polar Biol., 39, 583–592, https://doi.org/10.1007/s00300-015-1809-6, 2016.
Korthals, G. W., Van De Ende, A., Van Megen, H., Lexmond, T. M., Kammenga, J. E., and Bongers, T.: Short-term effects of cadmium, copper, nickel and zinc on soil nematodes from different feeding and life-history strategy groups, Appl. Soil Ecol., 4, 107–117, https://doi.org/10.1016/0929-1393(96)00113-8, 1996.
Łakomiec, P., Holst, J., Friborg, T., Crill, P., Rakos, N., Kljun, N., Olsson, P.-O., Eklundh, L., Persson, A., and Rinne, J.: Field-scale CH4 emission at a subarctic mire with heterogeneous permafrost thaw status, Biogeosciences, 18, 5811–5830, https://doi.org/10.5194/bg-18-5811-2021, 2021.
Lefcheck, J. S.: piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics, Methods Ecol. Evol., 7, 573–579, https://doi.org/10.1111/2041-210X.12512, 2016.
Lenth, R.: emmeans: Estimated Marginal Means, aka Least-Squares Means (Version 1.10.5) [R package], CRAN, https://rvlenth.github.io/emmeans/ (last access: 20 May 2025), 2024.
Li, Z. C., Sun, W. B., Liang, C. X., Xing, X. H., and Li, Q. X.: Arctic warming trends and their uncertainties based on surface temperature reconstruction under different sea ice extent scenarios, Adv. Clim. Change Res., 14, 335–346, https://doi.org/10.1016/j.accre.2023.06.003, 2023.
Liu, Y., Wang, W., Liu, P., Zhou, H., Chen, Z., and Suonan, J.: Plant–soil mediated effects of long-term warming on soil nematodes of alpine meadows on the Qinghai–Tibetan Plateau, Biology, 11, 1596, https://doi.org/10.3390/biology11111596, 2022.
Mackelprang, R., Saleska, S. R., Jacobsen, C. S., Jansson, J. K., and Taş, N.: Permafrost meta-omics and climate change, Annu. Rev. Earth Planet. Sci., 44, 439–462, https://doi.org/10.1146/annurev-earth-060614-105126, 2016.
Maharning, A. R., Widyastuti, A., and Pratiwi, M.: Soil bacteria and nematode functional diversity: A comparison across vegetation types, AIP Conf. Proc., 1744, 020004, https://doi.org/10.1063/1.4953478, 2016.
McCalley, C. K., Woodcroft, B. J., Hodgkins, S. B., Wehr, R. A., Kim, E.-H., Mondav, R., Crill, P. M., Chanton, J. P., Rich, V. I., Tyson, G. W., and Saleska, S. R.: Methane dynamics regulated by microbial community response to permafrost thaw, Nature, 514, 478–481, https://doi.org/10.1038/nature13798, 2014.
Mueller, C. W., Rethemeyer, J., Kao-Kniffin, J., Löppmann, S., Hinkel, K. M., and Bockheim, J. G.: Large amounts of labile organic carbon in permafrost soils of northern Alaska, Glob. Change Biol., 21, 2804–2817, https://doi.org/10.1111/gcb.12876, 2015.
Neher, D. A.: Ecology of plant and free-living nematodes in natural and agricultural soil, Annu. Rev. Phytopathol., 48, 371–394, https://doi.org/10.1146/annurev-phyto-073009-114439, 2010.
Newsham, K. K., Hall, R. J., and Maslen, N. R.: Experimental warming of bryophytes increases the population density of the nematode Plectus belgicae in Maritime Antarctica, Antarct. Sci., 33, 165–173, https://doi.org/10.1017/S0954102020000528, 2021.
Nielsen, U. N., Ayres, E., Wall, D. H., Li, G., Bardgett, R. D., Wu, T., and Garey, J. R.: Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties, Glob. Ecol. Biogeogr., 23, 968–978, https://doi.org/10.1111/geb.12177, 2014.
Obu, J.: How much of the Earth's surface is underlain by permafrost?, J. Geophys. Res.-Earth Surf., 126, e2021JF006123, https://doi.org/10.1029/2021JF006123, 2021.
Olvmo, M., Holmer, B., Thorsson, S., Reese, H., and Lindberg, F.: Sub-Arctic palsa degradation and the role of climatic drivers in the largest coherent palsa mire complex in Sweden (Vissátvuopmi), 1955–2016, Sci. Rep., 10, 8937, https://doi.org/10.1038/s41598-020-65719-1, 2020.
Otten, W., Hall, D., Harris, K., Ritz, K., Young, I. M., and Gilligan, C. A.: Soil physics, fungal epidemiology and the spread of Rhizoctonia solani, New Phytol., 151, 459–468, https://doi.org/10.1046/j.0028-646x.2001.00190.x, 2001.
Patzner, M. S., Logan, M., McKenna, A. M., Young, R. B., Zhou, Z., Joss, H., Mueller, C. W., Hoeschen, C., Scholten, T., Straub, D., Kleindienst, S., Borch, T., Kappler, A., and Bryce, C.: Microbial iron cycling during palsa hillslope collapse promotes greenhouse gas emissions before complete permafrost thaw, Commun. Earth Environ., 3, 1–14, https://doi.org/10.1038/s43247-022-00407-8, 2022.
Pedersen, A. R., Petersen, S. O., and Schelde, K.: A comprehensive approach to soil–atmosphere trace-gas flux estimation with static chambers, Eur. J. Soil Sci., 61, 888–902, https://doi.org/10.1111/j.1365-2389.2010.01291.x, 2010.
Poll, J., Marhan, S., Haase, S., Hallmann, J., Kandeler, E., and Ruess, L.: Low amounts of herbivory by root-knot nematodes affect microbial community dynamics and carbon allocation in the rhizosphere, FEMS Microbiol. Ecol., 62, 268–279, https://doi.org/10.1111/j.1574-6941.2007.00383.x, 2007.
Pothula, S. K., Phillips, G., and Bernard, E. C.: Increasing levels of physical disturbance affect soil nematode community composition in a previously undisturbed ecosystem, J. Nematol., 54, 20220022, https://doi.org/10.2478/jofnem-2022-0022, 2022.
R Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, http://www.R-project.org (last access: 20 May 2025), 2013.
Rantanen, M., Karpechko, A. Y., Lipponen, A., Nordling, K., Hyvärinen, O., Ruosteenoja, K., Vihma, T., and Laaksonen, A.: The Arctic has warmed nearly four times faster than the globe since 1979, Commun. Earth Environ., 3, 1–10, https://doi.org/10.1038/s43247-022-00498-3, 2022.
Romanovsky, V. E., Smith, S. L., and Christiansen, H. H.: Permafrost thermal state in the Polar Northern Hemisphere during the International Polar Year 2007–2009: A synthesis, Permafr. Periglac. Process., 21, 106–116, https://doi.org/10.1002/ppp.689, 2010.
Ruess, L., Michelsen, A., Schmidt, I. K., and Jonasson, S.: Simulated climate change affecting microorganisms, nematode density and biodiversity in subarctic soils, Plant Soil, 212, 63–73, 1999.
Sannel, A. B. K., Hugelius, G., Jansson, P., and Kuhry, P.: Permafrost warming in a subarctic peatland – which meteorological controls are most important, Permafrost Periglac. Process., 27, 177–188, https://doi.org/10.1002/ppp.1862, 2015.
Savin, M. C., Görres, J. H., Neher, D. A., and Amador, J. A.: Uncoupling of carbon and nitrogen mineralization: Role of microbivorous nematodes, Soil Biol. Biochem., 33, 1463–1472, https://doi.org/10.1016/S0038-0717(01)00055-4, 2001.
Schädel, C., Rogers, B. M., Lawrence, D. M., Koven, C. D., Brovkin, V., Burke, E. J., Genet, H., Huntzinger, D. N., Jafarov, E., McGuire, A. D., Riley, W. J., and Natali, S. M.: Earth system models must include permafrost carbon processes, Nat. Clim. Change, 14, 114–116, https://doi.org/10.1038/s41558-023-01909-9, 2024.
Schuur, E. A. G. and Abbott, B.: High risk of permafrost thaw, Nature, 480, 32–33, https://doi.org/10.1038/480032a, 2011.
Schuur, E. A. G., McGuire, A. D., Schädel, C., Grosse, G., Harden, J. W., Hayes, D. J., Hugelius, G., Koven, C. D., Kuhry, P., Lawrence, D. M., Natali, S. M., Olefeldt, D., Romanovsky, V. E., Schaefer, K., Turetsky, M. R., Treat, C. C., and Vonk, J. E.: Climate change and the permafrost carbon feedback, Nature, 520, 171–179, https://doi.org/10.1038/nature14338, 2015.
Schuur, E. A. G., Abbott, B. W., Commane, R., Ernakovich, J., Euskirchen, E., Hugelius, G., Grosse, G., Jones, M., Koven, C., Leshyk, V., Lawrence, D., Loranty, M. M., Mauritz, M., Olefeldt, D., Natali, S., Rodenhizer, H., Salmon, V., Schädel, C., Strauss, J., Treat, C., and Turetsky, M.: Permafrost and climate change: Carbon cycle feedbacks from the warming Arctic, Annu. Rev. Environ. Resour., 47, 343–371, https://doi.org/10.1146/annurev-environ-012220-011847, 2022.
Seppälä, M.: Synthesis of studies of palsa formation underlining the importance of local environmental and physical characteristics, Quaternary Res., 75, 366–370, https://doi.org/10.1016/j.yqres.2010.09.007, 2011.
Simmons, B. L., Wall, D. H., Adams, B. J., Ayres, E., Barrett, J. E., and Virginia, R. A.: Long-term experimental warming reduces soil nematode populations in the McMurdo Dry Valleys, Antarctica, Soil Biol. Biochem., 41, 2052–2060, https://doi.org/10.1016/j.soilbio.2009.07.009, 2009.
Singh, B. K., Bardgett, R. D., Smith, P., and Reay, D. S.: Microorganisms and climate change: Terrestrial feedbacks and mitigation options, Nat. Rev. Microbiol., 8, 779–790, https://doi.org/10.1038/nrmicro2439, 2010.
Singleton, C. M., McCalley, C. K., Woodcroft, B. J., Boyd, J. A., Evans, P. N., Hodgkins, S. B., Chanton, J. P., Frolking, S., Crill, P. M., Saleska, S. R., Rich, V. I., and Tyson, G. W.: Methanotrophy across a natural permafrost thaw environment, ISME J., 12, 2544–2558, https://doi.org/10.1038/s41396-018-0065-5, 2018.
Smith, T. E., Wall, D. H., Hogg, I. D., Adams, B. J., Nielsen, U. N., and Virginia, R. A.: Thawing permafrost alters nematode populations and soil habitat characteristics in an Antarctic polar desert ecosystem, Pedobiologia, 55, 75–81, https://doi.org/10.1016/j.pedobi.2011.11.001, 2012.
Sutton, L., Mueter, F. J., Bluhm, B. A., and Iken, K.: Environmental filtering influences functional community assembly of epibenthic communities, Front. Mar. Sci., 8, 736917, https://doi.org/10.3389/fmars.2021.736917, 2021.
Tianxiang, L., Huixin, L., Tong, W., and Feng, H.: Influence of nematodes and earthworms on the emissions of soil trace gases (CO2, N2O), Acta Ecol. Sin., 28, 993–999, https://doi.org/10.1016/S1872-2032(08)60033-5, 2008.
Tintori, S. C., Sloat, S. A., and Rockman, M. V.: Rapid isolation of wild nematodes by Baermann funnel, J. Vis. Exp., 179, e63287, https://doi.org/10.3791/63287, 2022.
Traunspurger, W., Bergtold, M., and Goedkoop, W.: The effects of nematodes on bacterial activity and abundance in a freshwater sediment, Oecologia, 112, 118–122, https://doi.org/10.1007/s004420050291, 1997.
Tromans, D.: Modeling oxygen solubility in water and electrolyte solutions, Ind. Eng. Chem. Res., 39, 805–812, https://doi.org/10.1021/ie990577t, 2000.
Tu, C., Koenning, S. R., and Hu, S.: Root-parasitic nematodes enhance soil microbial activities and nitrogen mineralization, Microb. Ecol., 46, 134–144, https://doi.org/10.1007/s00248-002-1068-2, 2003.
Van Daele, R.: Experimental warming and permafrost thaw decrease soil nematode abundance in northern palsa peatlands: all datasets and scripts, Web Ecology, R programming language (v4.2.2), Zenodo [code and data set], https://doi.org/10.5281/zenodo.15005319, 2025.
Van De Walle, R., Massol, F., Vandegehuchte, M. L., and Bonte, D.: The functional composition of dune nematode communities is structured by both biogeographic region and the local marram grass environment, Eur. J. Soil Biol., 117, 103511, https://doi.org/10.1016/j.ejsobi.2023.103511, 2023.
van den Hoogen, J., Geisen, S., Routh, D., Ferris, H., Traunspurger, W., Wardle, D. A., de Goede, R. G. M., Adams, B. J., Ahmad, W., Andriuzzi, W. S., Bardgett, R. D., Bonkowski, M., Campos-Herrera, R., Cares, J. E., Caruso, T., de Brito Caixeta, L., Chen, X., Costa, S. R., Creamer, R., da Cunha Castro, J. M., Dam, M., Djigal, D., Escuer, M., Griffiths, B. S., Gutiérrez, C., Hohberg, K., Kalinkina, D., Kardol, P., Kergunteuil, A., Korthals, G., Krashevska, V., Kudrin, A. A., Li, Q., Liang, W., Magilton, M., Marais, M., Rodríguez Martín, J. A., Matveeva, E., Mayad, E. H., Mulder, C., Mullin, P., Neilson, R., Nguyen, T. A. D., Nielsen, U. N., Okada, H., Palomares Rius, J. E., Pan, K., Peneva, V., Pellissier, L., Pereira da Silva, J. C., Pitteloud, C., Powers, T. O., Powers, K., Quist, C. W., Rasmann, S., Sánchez Moreno, S., Scheu, S., Setälä, H., Sushchuk, A., Tiunov, A. V., Trap, J., van der Putten, W., Vestergård, M., Villenave, C., Waeyenberge, L., Wall, D. H., Wilschut, R., Wright, D. G., Yang, J., and Crowther, T. W.: Soil nematode abundance and functional group composition at a global scale, Nature, 572, 194–198, https://doi.org/10.1038/s41586-019-1418-6, 2019.
Van Voorhies, W. A. and Ward, S.: Broad oxygen tolerance in the nematode Caenorhabditis elegans, J. Exp. Biol., 203, 2467–2478, https://doi.org/10.1242/jeb.203.16.2467, 2000.
Verschoor, B. C., Pronk, T. E., De Goede, R. G. M., and Brussaard, L.: Could plant-feeding nematodes affect the competition between grass species during succession in grasslands under restoration management?, J. Ecol., 90, 753–761, https://doi.org/10.1046/j.1365-2745.2002.00710.x, 2002.
Viketoft, M., Palmborg, C., Sohlenius, B., Huss-Danell, K., and Bengtsson, J.: Plant species effects on soil nematode communities in experimental grasslands, Appl. Soil Ecol., 30, 90–103, https://doi.org/10.1016/j.apsoil.2005.02.007, 2005.
Voigt, C., Virkkala, A. M., Gosselin, G. H., Bennett, K. A., Black, T. A., Detto, M., Chevrier-Dion, C., Guggenberger, G., Hashmi, W., Kohl, L., Kou, D., Marquis, C., Marsh, P., Marushchak, M. E., Nesic, Z., Nykänen, H., Saarela, T., Sauheitl, L., Walker, B., Weiss, N., Wilcox, E. J., and Sonnentag, O.: Arctic soil methane sink increases with drier conditions and higher ecosystem respiration, Nat. Clim. Change, 13, 1095–1104, https://doi.org/10.1038/s41558-023-01785-3, 2023.
Walvoord, M. A. and Kurylyk, B. L.: Hydrologic impacts of thawing permafrost – A review, Vadose Zone J., 15, vzj2016.01.0010, https://doi.org/10.2136/vzj2016.01.0010, 2016.
Wang, Y. R., Hessen, D. O., Samset, B. H., and Stordal, F.: Evaluating global and regional land warming trends in the past decades with both MODIS and ERA5-land land surface temperature data, Remote Sens. Environ., 280, 113181, https://doi.org/10.1016/j.rse.2022.113181, 2022.
Wild, J., Kopecký, M., Macek, M., Šanda, M., Jankovec, J., and Haase, T.: Climate at ecologically relevant scales: A new temperature and soil moisture logger for long-term microclimate measurement, Agric. Forest Meteorol., 268, 40–47, https://doi.org/10.1016/j.agrformet.2018.12.018, 2019.
Wu, Y., Chen, W., Entemake, W., Wang, J., Liu, H., Zhao, Z., Li, Y., Qiao, L., Yang, B., Liu, G., and Xue, S.: Long-term vegetation restoration promotes the stability of the soil micro-food web in the Loess Plateau in North-West China, Catena, 202, 105293, https://doi.org/10.1016/j.catena.2021.105293, 2021.
Wurst, S., Wagenaar, R., Biere, A., and van der Putten, W. H.: Microorganisms and nematodes increase levels of secondary metabolites in roots and root exudates of Plantago lanceolata, Plant Soil, 329, 117–126, https://doi.org/10.1007/s11104-009-0139-2, 2010.
Xu, Y. Y., Lu, H., Wang, X., Zhang, K. Q., and Li, G. H.: Effect of volatile organic compounds from bacteria on nematodes, Chem. Biodivers., 12, 1415–1421, https://doi.org/10.1002/cbdv.201400342, 2015.
Yeates, G. W.: Nematodes as soil indicators: Functional and biodiversity aspects, Biol. Fert. Soils, 37, 199–210, https://doi.org/10.1007/s00374-003-0586-5, 2003.
Yeates, G. W., Bongers, T., de Goede, R. G. M., Freckman, D. W., and Georgieva, S. S.: Feeding habits in soil nematode families and genera – an outline for soil ecologists, J. Nematol., 25, 315–331, 1993.
Yu, X., Song, C., Sun, L., Wang, X., Shi, F., Cui, Q., and Tan, W.: Growing season methane emissions from a permafrost peatland of northeast China: Observations using open-path eddy covariance method, Atmos. Environ., 153, 135–149, https://doi.org/10.1016/j.atmosenv.2017.01.026, 2017.
Yurkevich, M. G., Sushchuk, A. A., Matveeva, E. M., and Kalinkina, D. S.: Changes in soil nematode communities during post-agrogenic transformation of peat soils and vegetation, Eurasian Soil Sci., 53, 686–695, https://doi.org/10.1134/S1064229320050166, 2020.
Yvon-Durocher, G., Allen, A. P., Bastviken, D., Conrad, R., Gudasz, C., St-Pierre, A., Thanh-Duc, N., and del Giorgio, P. A.: Methane fluxes show consistent temperature dependence across microbial to ecosystem scales, Nature, 507, 488–491, https://doi.org/10.1038/nature13164, 2014.
Zaman, M., Kleineidam, K., Bakken, L., Berendt, J., Bracken, C., Butterbach-Bahl, K., Cai, Z., Chang, S. X., Clough, T., Dawar, K., Ding, W. X., Dörsch, P., dos Reis Martins, M., Eckhardt, C., Fiedler, S., Frosch, T., Goopy, J., Görres, C.-M., Gupta, A., Henjes, S., Hofmann, M. E. G., Horn, M. A., Jahangir, M. M. R., Jansen-Willems, A., Lenhart, K., Heng, L., Lewicka-Szczebak, D., Lucic, G., Merbold, L., Mohn, J., Molstad, L., Moser, G., Murphy, P., Sanz-Cobena, A., Šimek, M., Urquiaga, S., Well, R., Wrage-Mönnig, N., Zaman, S., Zhang, J., and Müller, C.: Direct and indirect effects of soil fauna, fungi and plants on greenhouse gas fluxes, Springer eBooks, 151–176, https://doi.org/10.1007/978-3-030-55396-8_5, 2021.
We studied the impact of climate change on nematodes in a palsa peatland in Norway. This ecosystem, crucial for carbon storage, is rapidly changing due to warming and permafrost thaw. We found that intact palsas host more nematode populations, but warming reduces their numbers, particularly bacterivores and omni-carnivores. Additionally, fungivores became more dominant over the summer. These changes may alter nutrient cycles, highlighting the need to study nematodes in fragile Arctic ecosystems.
We studied the impact of climate change on nematodes in a palsa...