the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Heat shock and plant leachates regulate seed germination of the endangered carnivorous plant Drosophyllum lusitanicum
Susana Gómez-González
Maria Paniw
Kamila Antunes
Fernando Ojeda
In fire-prone ecosystems, many plant species have specialized mechanisms of seed dormancy that ensure a successful recruitment after fire. A well-documented mechanism is the germination stimulated by fire-related cues, such as heat shock and smoke. However, less is known about the role of inhibitory germination signals (e.g. allelopathy) in regulating post-fire recruitment. Plant leachates derived from the unburned vegetation can enforce dormancy by means of allelopathic compounds, acting as a signal of unfavourable (highly competitive) niche for germination in pyrophyte species. Here, we assessed the separate effects of heat shock and plant leachates on seed germination of Drosophyllum lusitanicum, an endangered carnivorous plant endemic to Mediterranean fire-prone heathlands. We performed a germination experiment in which seeds were subjected to three treatments: (1) 5 min at 100 ∘C, (2) watering with plant leachate, and (3) control. Germination rate and seed viability was determined after 63 days. Heat shock stimulated seed germination in D. lusitanicum while plant leachates had inhibitory germination effects without reducing seed viability. Thus, both positive and negative signals could be involved in its successful post-fire recruitment. Fire would break seed dormancy and stimulate seed germination of D. lusitanicum through high temperatures, but also by eliminating allelochemical compounds from the soil. These results help to understand the population dynamics patterns found for D. lusitanicum in natural populations, and highlight the role of fire in the ecology and conservation of this endangered species. Seed dormancy imposed by plant-derived leachates as an adaptive mechanism should be considered more in fire ecology theory.
- Article
(701 KB) - Full-text XML
- BibTeX
- EndNote
Germination is a critical event in the life cycle of plants, since germinating under adverse conditions limits the chances of effective recruitment (i.e. survival and growth into adulthood) (Fenner and Thompson, 2005). Plants that have evolved under unpredictable environments exhibit seed traits that allow them to remain dormant and “recognize” cues associated with the favourable conditions that, in turn, trigger their germination (Finch-Savage and Leubner-Metzger, 2006; Willis et al., 2014). Temperature and light fluctuations, moisture, and/or the presence of certain chemicals are among the main environmental cues breaking seed dormancy and/or stimulating germination (Baskin and Baskin, 2014).
In fire-prone ecosystems, preeminent germination cues are provided by fire (Keeley et al., 2012). Heat shock and chemicals derived from smoke, ashes or charred wood are the cues often proposed by ecologists to test and illustrate fire-stimulated germination (e.g. Keeley, 1987; Moreira et al., 2010; Thomas et al., 2010). Fire-cued germination has been considered to be adaptive in fire-prone environments, since it makes seedling emergence coincide with temporary favourable conditions, where light and soil nutrients increase and negative biotic interactions (e.g. competition, seedling herbivory) are reduced (Bond and van Wilgen, 1996; Keeley and Fotheringham, 2000; Nelson et al., 2012), thus promoting effective recruitment. In addition, germinating late after a fire may prevent recruiting plants from reaching maturity before the next fire event, compromising their reproductive potential (McMaster and Zedler, 1981; Enright et al., 1998) and, hence, their fitness (de Luis et al., 2008).
On the other hand, less attention has been paid in the scientific literature to the role of negative signals, such as allelochemicals, that can also regulate post-fire seed germination (e.g. Keeley et al., 1985; Preston and Baldwin, 1999; Herranz et al., 2006). Many plant species are known to release secondary metabolites to the soil as leaf and litter leachates with allelopathic effects on seed germination (Richardson and Williamson, 1988; Hewitt and Menges, 2008; Li et al., 2010). For fire-adapted plants, this imposed seed dormancy by allelochemical leachates may be beneficial as they are indicative of non-favourable conditions for seedling emergence (high plant competition). Fire would then halt the release of allelopathic compounds by burning away plant and litter biomass, and fire-produced charcoal would adsorb allelopathic compounds from the soil (Zackrisson et al., 1996; Wardle et al., 1998; Keech et al., 2005). Thus, fire would also prompt germination by breaking seed dormancy after removing allelochemicals from the soil (e.g. Preston and Baldwin, 1999; Nelson et al., 2012).
Drosophyllum lusitanicum (L.) Link (Drosophyllaceae) is an endangered carnivorous plant species endemic to Mediterranean heathlands of the western Iberian Peninsula and northern Morocco (Correia and Freitas, 2002; Garrido et al., 2003; Paniw et al., 2015). These Mediterranean heathlands grow on nutrient-poor, acid soils, and are regarded as a “fire-adapted” vegetation type (Ojeda, 2009; Ojeda et al., 2010) in which recurrent natural wildfires have been present (at least) since the Pleistocene (Daniau et al., 2007; Keeley et al., 2012). The population dynamics of D. lusitanicum in natural heathland habitats are actually associated with recurrent fire (Paniw et al., 2017a). In a recent study, Cross et al. (2017) found that D. lusitanicum produces around 60–80 % of dormant seeds and germination is stimulated by heat shock (80 or 100 ∘C for 5–30 min) or after seed coat precision nicking. Their results also suggest that both smoke and light do not affect post-fire germination in this species (Cross et al., 2017).
In the absence of fire, D. lusitanicum disappears from the above-ground, dense heathland vegetation, but seeds persist in the soil seed bank until the next fire occurs or vegetation is removed by human-related activities (e.g. firebreaks, road sides, vegetation clearance for afforestation and forestry practices, cattle trampling) (Paniw et al., 2015, 2017a). That apparent association of D. lusitanicum with anthropogenic disturbance indicates that seed germination in this species might not only be affected by direct fire-related cues (e.g. heat shock; Cross et al., 2017) but also by indirect signals associated with the removal of plant (and plant litter) cover and their concomitant allelopathic effects. In fact, population growth rate in this species is highest when natural habitats are exposed to moderate levels of anthropogenic vegetation clearance in addition to fire (Paniw et al., 2017a). However, experimental studies comparing the effect of positive (e.g. heat shock) and negative (plant leachates) signals for seed germination in this singular species are lacking. Considering the endangered status of D. lusitanicum and the increasing frequency of anthropogenic disturbances affecting the Mediterranean heathlands (Paniw et al., 2015), understanding the germination ecology of this species will potentially help develop adequate management strategies for its conservation.
Here, we investigated the separate effects of heat shock and plant leachates on seed germination of D. lusitanicum in two different populations. As D. lusitanicum is a fire-adapted species that also colonizes open patches after vegetation clearing (Correia and Freitas, 2002; Paniw et al., 2015), we predicted that heat shock would stimulate seed germination, whereas plant leachates would inhibit it without having any further toxic effect on seed viability.
2.1 Study species
Drosophyllum lusitanicum is a rare, endangered carnivorous subshrub (∼40 cm high), with long, glandular trap leaves clustered in rosettes and showy, yellow flowers in stalked inflorescences (Salces-Castellano et al., 2016). It is endemic to the southwestern Iberian Peninsula (Europe) and the Tingitanian Peninsula (Africa), where it is restricted to fire-prone Mediterranean heathlands (Müller and Deil, 2001; Correia and Freitas, 2002), or human-disturbed heathland habitats, always on highly acidic, infertile soils (Paniw et al., 2015). In nature, germination occurs in late winter and plants reach sexual maturity in the second year after germination, flowering in mid–late spring and setting seeds in midsummer (Salces-Castellano et al., 2016). Seeds are black and pyriform (3 mm×2 mm approx.) with a relatively thick testa and a fully developed embryo at the moment of dispersal (Cross et al., 2017). They are water permeable and physiologically dormant, reaching only 20–40 % of final germination under optimal temperature conditions (15–20 ∘C) when not further treated (Cross et al., 2017).
2.2 Seed treatments and germination assay
To address the separate effects of heat shock and plant leachates on seed germination of D. lusitanicum, groups of seeds were allocated to one of three treatments: (1) 5 min at 100 ∘C and watering with distilled water (heat shock), (2) watering with plant leachate water (allelopathy), and (3) watering with distilled water (control). We decided not to consider a combined heat shock and plant leachate treatment because fire removes allelochemicals from the soil (Zackrisson et al., 1996; Wardle et al., 1998; Keech et al., 2005) before the first post-fire rains, so heat-shocked seeds in nature would not experience allelopathic effects. This design was replicated with seeds from two separate populations (Montera del Torero and Sierra Carbonera) located in Cádiz province, southwestern Spain (see Salces-Castellano et al., 2016, for location and detailed information of these populations), collected in July 2014, and stored in darkness with silica gel at room temperature until the germination experiment started in April 2016. Seeds were collected from randomly selected individuals (∼50) from each population. We used a randomized block design, using 25 seeds per block, and nine (Montera del Torero) or 10 blocks (Sierra Carbonera) per treatment, due to differences in seed availability between populations.
The heat shock treatment was performed in an oven, where seeds of each block were placed in separate aluminium trays and exposed to 100 ∘C during 5 min. All trays from each population were introduced in the oven at the same time in order to assure that they all were under the same conditions, since in our experience different heating events can produce noticeable temperature variations due to uncontrolled events (e.g. door opening). We assumed that there were no random events obscuring the effect of heat shock treatment as the oven was designed to distribute heat evenly (Morrison and Morris, 2000). Although the treatment was not applied on each sample independently (pseudoreplicated according to Morrison and Morris, 2000), seeds came from randomly selected individuals (as described above) and were randomly assigned to trays (block), which in turn, were randomly disposed within the oven. This way, we tried to maximize the independence of samples. Therefore, valid ecological inferences of the treatment effect can still be made (for a discussion on this topic see Oksanen, 2001, 2004; Hurlbert, 2004; Colegrave and Ruxton, 2017), particularly considering that our results were consistent with other studies on the ecology of D. lusitanicum in relation to fire (see discussion section). The heat shock dose was selected according to previous experimental work on seed germination in other Mediterranean pyrophytic species (e.g. Moreira et al., 2010), and based on the temperature observed in the soil during experimental fire in shrublands from other Mediterranean-type ecosystems (e.g. Gómez-González and Cavieres, 2009).
For the allelopathy treatment, we collected shoots of five common heathland shrub species – Calluna vulgaris, Erica australis, Halimium lasianthum, Cistus populifolius and Pterospartum tridentatum – abundant in the two sites where D. lusitanicum seeds were harvested (Müller and Deil, 2001; Garrido et al., 2003). After collection, we placed a 138 g (fresh weight) mixture of these plants in a flask, added 6 L of distilled water, and allowed the mixture to soak for 72 h. The resulting leachate (pH =5.98) was then filtered from the mixture, kept in the fridge, and used to water the seeds assigned to the allelopathy treatment during the germination assay.
Seeds of each block were placed in Petri dishes with filter paper and then randomly placed in a germination chamber under dark conditions (light does not affect seed germination of D. lusitanicum; Cross et al., 2017) and constant 18±2 ∘C temperature (germination of untreated seeds in this species is restricted to 15–20 ∘C; Cross et al., 2017). All Petri dishes were watered with distilled water, except those assigned to the allelopathy treatment, which were watered with the prepared leachate (see above). Petri dishes were watered and checked for seedling emergence every 1 or 2 days, and shifted around in the growth chamber to avoid possible positional biases and/or micro-environmental effects. Seeds were considered as germinated when the radicle was visible. The germination assay lasted for 63 days. After that, we determined the percentage of viable, non-germinated seeds in each treatment using the tetrazolium test (TTC 1 % in phosphate buffer, pH 7.3, and 24 h in darkness). This test was applied to 30 randomly selected non-germinated seeds from each treatment and population to explore (in a descriptive way) whether lack of germination in a given treatment was associated with seed mortality.
2.3 Statistical analyses
To determine the effect of the experimental treatment on the probability of germination (0, 1), we fitted generalized mixed effect models (GLMMs) with a binomial error distribution. Germination was described as a function of the fixed treatment effect, including heat, allelopathy, and control as treatment levels, and Petri dish was included as a random effect. The effect of treatment was tested with a likelihood ratio test (LRT; Vuong, 1989) and differences between treatment levels were tested by Wald-z test. Separate models were fitted for the two populations. All models were fitted using the lme4 package in R (Bates et al., 2015).
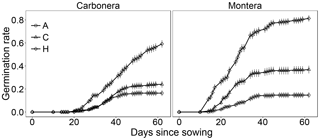
Figure 1Germination rate of D. lusitanicum seeds through time (days since sowing) in the experimental treatments. A: allelopathy; H: heat shock; C: control. Dots are mean values (n=9 in Montera del Torero and n=10 in Sierra Carbonera) and error lines are SE. Different letters represent significant pair-wise differences in the probability of germination (GLMM, Wald-z test, P<0.05, Table 1).
The probability of seed germination differed significantly among treatments in the two studied populations (Sierra Carbonera: χ2=119.2, d.f. =2, P<0.0001; Montera del Torero: χ2=225.1, d.f. =2, P<0.0001; LRT). In both populations, heat shock increased seed germination compared with the control treatment while plant leachates decreased it compared to control (Fig. 1, Table 1). The Montera del Torero population surpassed 80 % final germination under heat shock treatment, while the Sierra Carbonera population did not reach 60 % (Fig. 1). After the tetrazolium test, high values of seed viability of non-germinated seeds were found in almost all treatments and populations; 97 % in the control treatments of both populations, 97 and 90 % in the allelopathy treatments, and 90 and 76 % in the heat shock treatments of Montera del Torero and Sierra Carbonera, respectively. Therefore, only a moderate level of mortality (24 %) was detected in non-germinated seeds of Sierra Carbonera population after heat shock treatment.
Allelopathic effects are largely viewed as a mechanism of competitive ability, reducing the performance of co-occurring species (Hierro and Callaway, 2003). However, the inhibition of seed germination by allelochemical leachates from the aboveground vegetation (or litter) would exert positive effects on fitness in fire-adapted plant species by precluding seedling emergence into an unfavourable, highly competitive, pre-fire environment (Preston and Baldwin, 1999). In this sense, the role of plant chemical leachates in imposing seed dormancy (e.g. Richardson and Williamson, 1988; Hewitt and Menges, 2008) and their temporary elimination from the soil by fire (Wardle et al., 1998), thereby favouring post-fire recruitment, was nicely demonstrated by Preston and Baldwin (1999) in the post-fire annual Nicotiana attenuata. However, the potential relevance of plant chemical leachates in controlling post-fire seed germination has been rather overlooked in the fire ecology literature (e.g. Keeley et al., 2011, 2012).
The results of our experimental study showed that while seed germination in D. lusitanicum was stimulated by heat shock, it was inhibited by plant leachates without significantly reducing seed viability. Thus, both positive (germination cues) and negative (imposed dormancy) signals might be involved in modulating post-fire recruitment in this pyrophyte species. Although the leachate concentration used in our experimental design might not match the natural soil concentrations in the field, seed viability was not appreciably affected, indicating that there are secondary metabolite compounds in the plant leachate enforcing seed dormancy in D. lusitanicum. Consistently, Paniw et al. (2017c) experimentally demonstrated that seed germination of D. lusitanicum was inhibited under shrubs of unburned Mediterranean heathlands when compared to adjacent burned areas. It is known that ericaceous vegetation from other heathland communities can release phenolic compounds that inhibit germination of pyrogenic species (Zackrisson et al., 1996; Wardle et al., 1998). Fire-produced charcoal would then adsorb those compounds from the soil (Zackrisson et al., 1996; Wardle et al., 1998; Keech et al., 2005), thus relaxing or suppressing the imposed dormancy effects.
Our results help to understand the population dynamics of D. lusitanicum in natural habitats, where the combination of fire and moderate levels of anthropogenic vegetation clearance ensures population persistence (Paniw et al., 2017a). Paniw et al. (2015) established that, in the absence of fire, local populations of this species may remain viable in highly disturbed heathland patches as long as human-related activities (e.g. firebreaks, forestry practices, cattle browsing and trampling) remove heathland vegetation. This could alleviate the leachate-imposed seed dormancy and, thus, allow some continuous seed germination in anthropized habitats. Although the influence of high summer soil temperatures after vegetation removal on seed germination might also be suggested (Moreira and Pausas, 2012), we consider that this is unlikely since mean summer temperatures 1 m above the ground in the study sites do not reach 30 ∘C (Paniw, 2016), and maximum temperatures rarely exceed 40 ∘C (data not shown). In any case, vegetation removal alone does not produce the massive emergence of D. lusitanicum seedlings observed after fire in natural populations (Paniw et al., 2017a, b). Fire-related heat shock is still needed to stimulate seed germination and ensure a successful recruitment into a favourable post-fire habitat, which is key for the long-term population persistence of this endangered carnivorous plant (Paniw et al., 2017a, b, c). Indeed, the extinction risk of D. lusitanicum populations in highly disturbed, fire-free habitats is high due to seed bank depletion (Paniw et al., 2017a). Therefore, although D. lusitanicum does not have a strict fire-dependent recruitment, germination is fire stimulated and populations are better able to persist in the presence of fire (i.e. it is a fire-adapted species; Paniw et al., 2015, 2107a). Recurrent fires also favour recruitment and hence population persistence in other carnivorous plant species (Brewer, 2001; Cross et al., 2013), so fire ecology research may provide valuable insights into the conservation of this singular group of plants.
We found some differences between the studied populations in their seed germination and mortality after heat shock treatment. The Montera del Torero population reached higher final germination (∼80 %) and lower seed mortality (10 %) than the Sierra Carbonera population (∼60 % germination and 24 % mortality). This could be related to differences in their seed traits. In a previous study conducted on the same populations, Salces-Castellano et al. (2016) showed that plants from Montera del Torero had smaller seeds than plants from Sierra Carbonera. Differences in fire regimes and evolutionary processes among populations can be responsible for variations in seed traits, shaping adaptive responses to fire (Gómez-González et al., 2011, 2016), and may therefore explain, at least in part, the differences in germination patterns between the two populations. Several studies have related small seed size to better post-fire performance (e.g. Hanley et al., 2003; Shryock et al., 2014). However, the relationship between seed size and post-fire germination depends on the species and the ecological context (e.g. Gómez-González et al., 2011; Tavşanoğlu and Çatav, 2012). Future studies should address whether contrasting population dynamics in this species as a consequence of alterations of its fire-related life cycle (Paniw et al., 2017a) are accompanied by evolutionary changes in seed traits such as seed size.
In summary, heat shock and plant leachates have antagonistic effects on seed germination of D. lusitanicum, suggesting that both positive and negative signals can be involved in regulating post-fire seedling recruitment of this endangered species. Fire would favour seed germination of D. lusitanicum by means of two additive mechanisms: (i) the dormancy breaking by heat shock, and (ii) the release of plant-leachate-imposed dormancy by vegetation and litter burning. The occurrence of local populations established on highly disturbed heathland habitats (with very low shrub vegetation cover) in absence of fire might be explained, in part, by the reduction of the inhibitory effects of plant leachates. Our results contribute to improve the knowledge on the germination ecology of this endangered species and suggest that plant leachates from unburned vegetation could have a relevant role in regulating post-fire recruitment of fire-adapted plant species. We propose that allelopathy should be more considered in the fire ecology literature.
Raw data are available at https://figshare.com/s/23e0d4aa93d4d5f1e8f0 (Gómez-González et al., 2017).
The supplement related to this article is available online at: https://doi.org/10.5194/we-18-7-2018-supplement.
FO and MP conceived the idea; KA performed the experiments; SG-G and MP performed the statistical analyses and graphs; SG-G wrote the original draft; and SG-G, FO, and MP edited and reviewed subsequent drafts.
The authors declare that they have no conflict of interest.
The Andalusian Consejería de Medio Ambiente provided the necessary
permits to work with Drosophyllum lusitanicum, an endemic, red-listed species. Financial support has
been provided by project BREATHAL (CGL2011-28759/BOS, Spanish Ministerio de
Ciencia e Innovación) and project HERRIZA (CGL2015-64007-P,
MINECO-FEDER, Spain). Susana Gómez-González was partially funded by FONDAP/CONICYT-15110009
(Chile). Maria Paniw was supported by an FPI scholarship from the Spanish Ministerio
de Economía y Competitividad by an ERC Starting Grant (#337785).
Edited by: Hermann Heilmeier
Reviewed by: two anonymous referees
Baskin, C. C. and Baskin, J. M.: Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, Academic Press, San Diego, USA, 1600 pp., 2014.
Bates, D., Maechler, M., Bolker, B., and Walker, S.: Fitting linear mixed-effects models using lme4, J. Stat. Softw., 67, 1–48, 2015.
Bond, W. J. and van Wilgen, B. W.: Fire and Plants, Chapman and Hall, London, UK, 263 pp., 1996.
Brewer, J. S.: A demographic analysis of fire-stimulated seedling establishment of Sarracenia alata (Sarraceniaceae), Am. J. Bot., 88, 1250–1257, 2001.
Colegrave, N. and Ruxton, G. D.: Using biological insight and pragmatism when thinking about pseudoreplication, Trends Ecol. Evol., 33, 28–35, 2018.
Correia, E. and Freitas, H.: Drosophyllum lusitanicum, an endangered West Mediterranean endemic carnivorous plant: threats and its ability to control available resources, Bot. J. Linn. Soc., 140, 383–390, 2002.
Cross, A. T., Merritt, D. J., Turner, S. R., and Dixon, K. W.: Seed germination of the carnivorous plant Byblis gigantea (Byblidaceae) is cued by warm stratification and karrikinolide, Bot. J. Linn. Soc., 173, 143–152, 2013.
Cross, A. T., Paniw, M., Ojeda, F., Turner, S. R., Dixon, K. W., and Merritt, D. J.: Defining the role of fire in alleviating seed dormancy in a rare Mediterranean endemic subshrub, AoB Plants, 9, plx036, https://doi.org/10.1093/aobpla/plx036, 2017.
Daniau, A.-L., Sánchez-Goñi, M. F., Beaufort, L., Laggoun-Défarge, F., Loutre, M.-F., and Duprat, J.: Dansgaard–Oeschger climatic variability revealed by fire emissions in southwestern Iberia, Quaternary Sci. Rev., 26, 1369–1383, 2007.
de Luis, M., Verdú, M., and Raventós, J.: Early to rise makes a plant healthy, wealthy, and wise, Ecology, 89, 3061–3071, 2008.
Enright, N. J., Marsula, R., Lamont, B. B., and Wissel, C.: The ecological significance of canopy seed storage in fire-prone environments: a model for non-sprouting shrubs, J. Ecol., 86, 946–959, 1998.
Fenner, M. and Thompson, K.: The Ecology of Seeds, Cambridge University Press, Cambridge, UK, 260 pp., 2005.
Finch-Savage, W. E. and Leubner-Metzger, G.: Seed dormancy and the control of germination, New Phytol., 171, 501–523, 2006.
Garrido, B., Hampe, A., Marañón, T., and Arroyo, J.: Regional differences in land use affect population performance of the threatened insectivorous plant Drosophyllum lusitanicum (Droseraceae), Divers. Distrib., 9, 335–350, 2003.
Gómez-González, S. and Cavieres, L. A.: Litter burning does not equally affect seedling emergence of native and alien species of the Mediterranean-type Chilean matorral, Int. J. Wildland Fire, 18, 213–221, 2009.
Gómez-González, S., Torres-Díaz, C., Bustos-Schindler, C., and Gianoli, E.: Anthropogenic fire drives the evolution of seed traits, P. Natl. Acad. Sci. USA, 108, 18743–18747, 2011.
Gómez-González, S., Ojeda, F., Torres-Morales, P., and Palma, J. E.: Seed pubescence and shape modulate adaptive responses to fire cues, PLoS One, 11, e0159655, https://doi.org/10.1371/journal.pone.0159655, 2016.
Gómez-González, S., Paniw, M., Antunes, K., and Ojeda, F.: Raw data Gómez-González et al. Web Ecology, available at: https://figshare.com/s/23e0d4aa93d4d5f1e8f0, 2017.
Hanley, M. E., Unna, J. E., and Darvill, B.: Seed size and germination response: a relationship for fire-following plant species exposed to thermal shock, Oecologia, 134, 18–22, 2003.
Herranz, J. M., Ferrandis, P., Copete, M. A., Duro, E. M., and Zalacain, A.: Effect of allelopathic compounds produced by Cistus ladanifer on germination of 20 Mediterranean taxa, Plant Ecol., 184, 259–272, 2006.
Hewitt, R. E. and Menges, E. S.: Allelopathic effects of Ceratiola ericoides (Empetraceae) on germination and survival of six Florida scrub species, Plant Ecol., 198, 47–59, 2008.
Hierro, J. L. and Callaway, R. M.: Allelopathy and exotic plant invasion, Plant Soil, 256, 29–39, 2003.
Hurlbert, S. H.: On misinterpretations of pseudoreplication and related issues: a reply to Oksanen, Oikos, 104, 591–597, 2004.
Keech, O., Carcaillet, C., and Nilsson, M.-C.: Adsorption of allelopathic compounds by wood-derived charcoal: the role of wood porosity, Plant Soil, 272, 291–300, 2005.
Keeley, J. E.: Role of fire in seed germination of woody taxa in California chaparral, Ecology, 68, 434–443, 1987.
Keeley, J. E. and Fotheringham, C. J.: Role of fire in regeneration from seed, in: Seeds: The Ecology of Regeneration in Plant Communities, edited by: Fenner, M., CAB International, Oxon, UK, 311–330, 2000.
Keeley, J. E., Morton, B. A., Pedrosa, A., and Trotter, P.: Role of allelopathy, heat and charred wood in the germination of chaparral herbs and suffrutescents, J. Ecol., 73, 445–58, 1985.
Keeley, J. E., Pausas, J. G., Rundel, P. W., Bond, W. J., and Bradstock, R. A.: Fire as an evolutionary pressure shaping plant traits, Trends Plant Sci., 16, 406–411, 2011.
Keeley, J. E., Bond, W. J., Bradstock, R. A., Pausas, J. G., and Rundel, P. W.: Fire in Mediterranean Ecosystems: Ecology, Evolution and Management, Cambridge University Press, Cambridge, UK, 515 pp., 2012.
Li, Z.-H., Wang, Q., Ruan, X., Pan, C.-D., and Jiang, D.-A.: Phenolics and plant allelopathy, Molecules, 15, 8933–8952, 2010.
McMaster, G. S. and Zedler, P. H.: Delayed seed dispersal in Pinus torreyana (Torrey pine), Oecologia, 51, 62–66, 1981.
Moreira, B. and Pausas, J. G.: Tanned or burned: the role of fire in shaping physical seed dormancy, PLoS One, 7, e51523, https://doi.org/10.1371/journal.pone.0051523, 2012.
Moreira, B., Tormo, J., Estrelles, E., and Pausas, J. G.: Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora, Ann. Bot., 105, 627–635, 2010.
Morrison, D. A. and Morris, E. C.: Pseudoreplication in experimental designs for the manipulation of seed germination treatments, Austral. Ecol., 25, 292–296, 2000.
Müller, J. and Deil, U.: Ecology and structure of Drosophyllum lusitanicum (L.) Link populations in the south-west of the Iberian Peninsula, Acta Bot. Malac., 26, 47–68, 2001.
Nelson, D. C., Flematti, G. R., Ghisalberti, E. L., Dixon, K. W., and Smith, S. M.: Regulation of seed germination and seedling growth by chemical signals from burning vegetation, Annu. Rev. Plant Biol., 63, 107–30, 2012.
Ojeda, F.: 4030 Brezales secos europeos, in: Bases Ecológicas Preliminares para la Conservación de los Tipos de Hábitat de Interés Comunitario en España, auct.pl., Dirección General de Medio Natural – Ministerio de Medio Ambiente, y Medio Rural y Marino, Madrid, España, 1–66, 2009.
Ojeda, F., Pausas, J. G., and Verdú, M.: Soil shapes community structure through fire, Oecologia, 163, 729–735, 2010.
Oksanen, L.: Logic of experiments in ecology: is “pseudoreplication” a pseudoissue?, Oikos, 94, 27–28, 2001.
Oksanen, L.: The devil lies in details: reply to Stuart Hurlbert, Oikos, 104, 598–605, 2004.
Paniw, M. S.: Demography and Evolutionary Ecology of the Carnivorous Subshrub Drosophyllum lusitanicum (L.) Link (Drosophyllaceae). Ph.D. thesis, Universidad de Cádiz, Spain, 238 pp., 2016.
Paniw, M., Salguero-Gómez R., and Ojeda, F.: Local-scale disturbances can benefit an endangered, fire-adapted plant species in Western Mediterranean heathlands in the absence of fire, Biol. Conserv., 187, 74–81, 2015.
Paniw, M., Quintana-Ascencio, P., Ojeda, F., and Salguero-Gómez, R.: Interacting livestock and fire may both threaten and increase viability of a fire-adapted Mediterranean carnivorous plant. J. Appl. Ecol., 54, 1884–1894, 2017a.
Paniw, M., Quintana-Ascencio, P., Ojeda, F., and Salguero-Gómez, R.: Accounting for uncertainty in dormant life stages in stochastic demographic models, Oikos, 126, 900–909, 2017b.
Paniw, M., Salguero-Gómez, R., and Ojeda, F.: Transient facilitation of resprouting shrubs in fire-prone habitats, J. Plant. Ecol., https://doi.org/10.1093/jpe/rtx019, in press, 2017c.
Preston, C. A. and Baldwin, I. T.: Positive and negative signals regulate germination in the post-fire annual Nicotiana attenuata, Ecology, 80, 481–494, 1999.
Richardson, D. R. and Williamson, G. B.: Allelopathic effects of shrubs of the sand pine scrub on pines and grasses of sandhills, Forest Sci., 34, 592–605, 1988.
Salces-Castellano, A., Paniw, M., Casimiro-Soriguer, R., and Ojeda, F.: Attract them anyway – Benefits of large, showy flowers in a highly autogamous, carnivorous plant species, AoB Plants, 8, plw017, https://doi.org/10.1093/aobpla/plw017, 2016.
Shryock, D. F., DeFalco, L. A., and Esque, T. C.: Life-history traits predict perennial species response to fire in a desert ecosystem, Ecol. Evol., 4, 3046–3059, 2014.
Tavşanoğlu, Ç., and Çatav, Ş. S.: Seed size explains within-population variability in post-fire germination of Cistus salviifolius, Ann. Bot. Fenn., 49, 331–340, 2012.
Thomas, P. B., Morris, E. C., Auld, T. D., and Haigh, A. M.: The interaction of temperature, water availability and fire cues regulates seed germination in a fire-prone landscape, Oecologia, 162, 293–302, 2010.
Vuong, H. Q.: Likelihood ratio tests for model selection and non-nested hypotheses, Econometrica, 57, 307–333, 1989.
Wardle, D. A., Zackrisson, O., and Nilsson, M.-C.: The charcoal effect in Boreal forests: mechanisms and ecological consequences, Oecologia, 115, 419–426, 1998.
Willis, C. G., Baskin, C. C., Baskin, J. M., Auld, J. R., Venable, D. L., Cavender-Bares, J., Donohue, K., Rubio de Casas, R., and NESCent Germination Working Group: The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants, New Phytol., 203, 300–309, 2014.
Zackrisson, O., Nilsson, M.-C., and Wardle, D.: Key ecological function of charcoal from willdfire in the Boreal forest, Oikos, 77, 10–19, 1996.






