the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Insights into the habitat associations, phylogeny, and diet of Pipistrellus maderensis in Porto Santo, northeastern Macaronesia
Nia Toshkova
Angelina Gonçalves
André Reis
Elena J. Soto
Sergio Puertas Ruiz
Vanessa A. Mata
Catarina Rato
Ricardo Rocha
Around 60 % of all bat species occur in islands, and nearly one in four is an insular endemic. Bats are often the only native terrestrial mammals in oceanic islands, and despite increasing anthropogenic pressures, little is known about the distribution, natural history, and population status of most insular bat populations. The sub-tropical archipelago of Madeira is composed of the volcanic islands of Madeira, Porto Santo, and Desertas and is home to the Macaronesian endemic Pipistrellus maderensis, to the endemic subspecies Nyctalus leisleri verrucosus, and to Plecotus austriacus. Pipistrellus maderensis is known to both Madeira and Porto Santo, whereas the other two species have only been recorded in the former. However, no bats have been recorded in Porto Santo for over 15 years, raising fears that bats are probably extinct in the island. In July 2021, we conducted an island-wide acoustic survey using AudioMoth passive acoustic recorders, leading to the detection of Pipistrellus maderensis in 28 out of the 46 sampling sites (60 %). The species' activity was strongly associated with artificial water sources, and genetic samples from six captured individuals revealed that the populations of Pipistrellus maderensis in Porto Santo and Madeira have a close phylogenetic affinity. Furthermore, using DNA metabarcoding, we found that the species feeds on a wide variety of insects, including several economically important pest species and disease vectors. These findings emphasise the need to target more conservation and research efforts towards extant island bat populations and the potential ecosystem services they provide.
- Article
(3924 KB) - Full-text XML
-
Supplement
(366 KB) - BibTeX
- EndNote
The unique size, isolation, and microclimatic/geomorphological conditions of oceanic islands often lead to significant evolutionary divergence and high rates of endemism (Emerson, 2002; Kier et al., 2009). They often act as evolutionary reservoirs for lineages that have disappeared elsewhere and, as such, are crucial for the fate of biodiversity in the Anthropocene (Russell and Kueffer, 2019; Nori et al., 2022). However, historical and ongoing human-induced environmental degradation have resulted in acute changes to island biotas (Nogué et al., 2021). Consequently, ca. 50 % of the planet's terrestrial vertebrates are island dwellers (Leclerc et al., 2018), and most recorded extinctions since the expansion of Europeans have occurred in islands (Blackburn et al., 2004; Fernández-Palacios et al., 2021; Matthews et al., 2022).
Of over 1400 currently recognised bat species, ca. 25 % are island endemics (Conenna et al., 2017). Of these, over 60 % of the bats currently assessed by the International Union for Conservation of Nature (IUCN) are species formerly classified as “microbats”, i.e. laryngeal echolocating bats (Conenna et al., 2017; Frick et al., 2020). Similarly to their mainland counterparts, some island-dwelling microbats are seed dispersers and pollinators (Ramirez-Francel et al., 2021). However, most species are insectivores and potentially play pivotal roles in the suppression of arthropods, including agricultural pests and mosquitoes (Kemp et al., 2019). More than one-fourth of these laryngeal echolocating bats are assessed as CR, EN, or VU by the IUCN Red List, largely due to the additive and often synergistic effects of habitat loss and fragmentation, as well as the impacts of invasive species (Rodríguez-Durán et al., 2010; Conenna et al., 2017).
The Macaronesian biogeographical region, composed of the archipelagos of the Azores, Madeira, Selvagens Islands, Canary Islands, and Cabo Verde, is home to at least 15 bat species, including three island-restricted ones, namely the Azores noctule Nyctalus azoreum, the Canary big-eared bat Plecotus teneriffae, and the Madeiran pipistrelle Pipistrellus maderensis (Gonzáles-Dionis et al., 2022). The latter is a relatively small bat (forearm length = 29.5–34.0 mm) likely derived from African Pipistrellus kuhlii that colonised the Canary Islands and the archipelago of Madeira (Pestano et al., 2003) or from a common ancestor to both species (Jesus et al., 2013). It is a synanthropic species with flexible habitat requirements, being found in the archipelagos of Madeira, the Canary Islands, and possibly Azores (Trujillo and Gonzalez, 2011; Rainho, 2022; Rocha, 2021; Rainho et al., 2002). The species seems to be more abundant at lower altitudes, occurring in a variety of natural and humanised habitats, such as native forests and agricultural and urban areas (Teixeira and Jesus, 2009; Jesus et al., 2009; Ferreira et al., 2022; Nouioua, 2022; Rocha, 2021). It feeds on a wide diversity of arthropods (Gonçalves, 2022) and is prone to roost in cliffs, tree holes, and a variety of human-made structures such as bridges and tunnels (Rocha, 2021).
Pipistrellus maderensis is listed by the IUCN as vulnerable (Alcalde and Juste, 2016) and is one of Europe's most threatened bat species. Its geographic isolation and fragmented populations, typical of insular species, make it particularly vulnerable to anthropogenic stressors and natural catastrophes (Rocha, 2021). The threats to the conservation of aerial insectivorous bats such as Pipistrellus spp. are numerous, and since they are small, mostly nocturnal, and inconspicuous, their population declines often go unnoticed. As an extreme example, the last known Christmas Island pipistrelle Pipistrellus murrayi disappeared in 2009, becoming the first animal to go extinct in Australia in the last 5 decades (Woinarski, 2018). In Madeira, where it is more abundant, the population was suggested to be smaller than 1000 individuals (Cabral et al., 2005), whereas Azores might be home to fewer than 300 individuals (Queiroz et al., 2005).
Although Pipistrellus maderensis were known to occur in Porto Santo (archipelago of Madeira), previous surveys failed to detect the species (Jesus et al., 2013). However, it is unclear if the method and intensity of the surveys were sufficient to prove the absence of the species in the island. Here, we combine bioacoustics, phylogenetic analysis, and DNA metabarcoding to investigate the population status, evolutionary history, and trophic interactions of Pipistrellus maderensis in Porto Santo. Specifically, we address the following questions.
-
Is Porto Santo still home to an extant population of Pipistrellus maderensis? We anticipate that Pipistrellus maderensis still persists in Porto Santo, and we predict that considering the xeric climate of the island, bat activity is likely to be strongly influenced by artificial water sources.
-
What is the phylogenetic relationship between the populations of Pipistrellus maderensis from Madeira and Porto Santo? We hypothesise that both populations should be closely related, and considering the relatively small distance between Madeira and Porto Santo (ca. 40 km), bat populations in both islands will likely exhibit low genetic divergence.
-
Is Pipistrellus maderensis preying on agricultural insect pests in Porto Santo? We expect bats to prey mainly on moths and Diptera, and we predict that some of these will be agricultural pests.
2.1 Study area
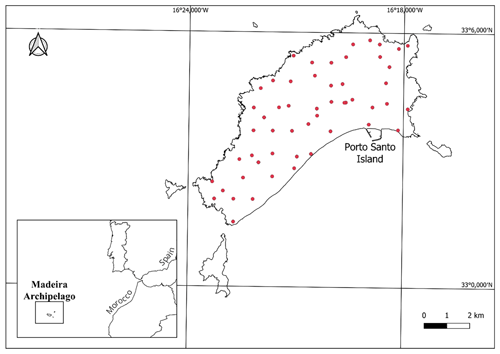
Porto Santo (ca. 42 km2) is the second largest and the oldest (∼ 14 million years) island of the Portuguese archipelago of Madeira, located in the Atlantic Ocean, near the coast of North Africa (Fig. 1; 38∘40 N, 27∘13 W). It has a Mediterranean xeric oceanic bioclimate, influenced by the Azores anticyclone (Rivas-Martínez, 2009). Despite some steep slopes, especially in the eastern section, the island is relatively flat, with the highest peak being Pico do Facho (517 m a.s.l.) (Kratochwil and Schwabe, 2018). Largely due to these geomorphological features, human intervention has been widespread throughout the island since the arrival of the Portuguese in the XV century, and Porto Santo's native vegetation is nowadays restricted to small, localised patches. Non-native coniferous species (mostly pine and/or cypress) were planted in several of the island's peaks to prevent soil erosion (Sparrius et al., 2017). Notwithstanding its small size, the island harbours a wide diversity of land-use covers – e.g. urban areas, agricultural fields, grasslands, and forests – and despite considerable environmental degradation, it is still home to nearly 250 extant endemic taxa, such as the IUCN critically endangered vetch Vicia ferreirensis (Carvalho and Osborne, 2011). As with numerous other oceanic islands, human arrival was accompanied by the introduction of several exotic vertebrates, among which are the domestic cat Felis catus, the European rabbit Oryctolagus cuniculus, and house mice Mus musculus (Borges et al., 2008; Rocha et al., 2017).
2.2 Bat surveys
We conducted an island-wide bioacoustic survey in June 2021. Contingent to orography, we sampled 55 randomly selected sites (generated using the random points tool in QGIS), spaced ca. 1 km apart throughout Porto Santo. However, only 46 sites were used in the analysis due to recorder failures – see below. In each sampling site, bats were surveyed for one night, using an AudioMoth recorder (Hill et al., 2018) placed within an appropriate waterproof box and attached to a tree trunk or a shrub. Each detector was configured to record continuously from half an hour before sunset to half an hour after sunrise, at a sample rate of 250 kHz.
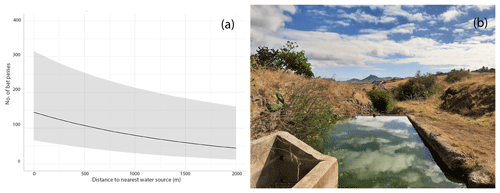
Figure 2Relationship between bat activity (number of bat passes) and distance to the nearest water source (a); artificial water point where intense bat activity was detected (b).
Additionally, we conducted four capture sessions using ground-level mist nets placed in potential foraging and drinking sites, such as forest trails, tunnel exits, and water ponds, and undertook targeted searches in a vast array of potential roosts – e.g. caves, abandoned houses, and underground pipelines. From each captured individual, a small wing tissue sample (< 2 mm in diameter) was collected using a biopsy punch and later preserved in 96 % ethanol. The age of each specimen was determined by examination of the extent of ossification in the epiphyses of the phalanges, and, in the case of adult females, the reproductive state was recorded by palpation (pregnant vs. non pregnant) and evidence of hair loss around the mamma and of milk leftovers/or production (lactating vs. non lactating). Additionally, whenever possible, guano pellets were collected from clean holding bags and stored with silica gel. Bat capture and handling was performed following recommendations approved by the American Society of Mammalogists (Sikes et al., 2011), and all bats were released at the capture site. No bioacoustic surveys or captures were conducted on rainy or windy days, and precipitation and wind speed were fairly constant throughout the sampling period.
2.3 Bioacoustic analysis
Using Kaleidoscope v. 5.3 software (Wildlife Acoustics, USA), the AudioMoth recordings in WAV format were split into 5 s recordings. If two or more bat pulses were detected in a 5 s recording, this was defined as a “bat pass”, which we used as our unit of measure for bat activity (see e.g. López-Bosch et al., 2022; Yoh et al., 2022). Kaleidoscope was programmed to detect the signals in the recordings with frequency ranges between 8 and 120 kHz and the pulse length between 1 and 500 ms. We ran the automated bat identification in Kaleidoscope Pro and manually verified the sound pulses in the sonograms. The identification of the echolocation recordings followed Teixeira and Jesus (2009).
2.4 Bat–environment relationships
Landscape-scale land-use metrics were acquired from 25 ha spatial land-use maps based on CORINE Land Cover 2018. The major land-use types in Porto Santo were defined as forest, agriculture, grassland, non-vegetated areas, and built-up areas (Table S1 in the Supplement). Previous assessments investigating the effect of landscape-scale land-use metrics on the activity of Pipistrellus maderensis at three different sizes (250, 500, and 1000 m) revealed consistent responses across scales (Ferreira et al., 2022). Thus, considering the small extension of Porto Santo (maximum length of ca. 9 km) and so to minimise the spatial overlap between neighbouring buffers, we used QGIS v. 3.28.0 to calculate the area of each land-use type inside buffers of 250 m, centred in each sampling site. Additionally, we used Google Earth complemented with field validation to determine the distance between sampling sites and the closest water sources (Table S2).
The effects of land-use type on bat activity (number of bat passes per night) was assessed using generalised linear mixed models (GLMMs) with a negative binomial distribution. As severe collinearity between predictor variables can undermine model inference (Dormann et al., 2013), prior to GLMMs, explanatory variables were centred and scaled (x=0, σ=1). We, therefore, quantified collinearity using variance inflation factors (VIFs), and variables with VIF ≥ 3 were excluded. To account for the nested spatiotemporal sampling design, sampling day was considered as a random factor in the GLMMs. A candidate model set was further constructed using all additive combinations of the four explanatory variables retained, and models were ranked based on Akaike information criterion adapted for small samples (AICc), using the MuMIn R package (Bartoń, 2020). To account for model uncertainty in multi-model inference, model averaging was used to obtain parameter estimates from the most plausible models (i.e. 0< ΔAICc < 2) (Burnham and Anderson, 2002). All GLMMs were conducted using R v. 4.2.1 software and the glmmTMB package (Brooks et al., 2017).
2.5 Phylogenetic analyses
Genomic DNA was extracted from wing tissue samples of six Pipistrellus maderensis captured during mist netting, using the E.Z.N.A Tissue DNA Kit (Mag-Bind). A fragment of the cytochrome b (cytb) gene was amplified by polymerase chain reaction (PCR) using the primers Molcit-F (Ibáñez et al., 2006) and MVZ-16 (Smith and Patton, 1993). Amplification of the cytb fragment was carried out in a 10 µL volume, comprised of 5 µL of QIAGEN Multiplex PCR Master Mix (Quiagen, Crawley, UK), 0.3 µL of each primer, 3.4 µL of ultra-pure water, and 2 µL of DNA extract. The PCR cycling procedure was done according to Ibáñez et al. (2006). All amplified fragments were sequenced in a Sanger sequencer and deposited to the GenBank database, with accession numbers from OQ260001 to OQ260006 (Table S3).
A total of 395 cytb sequences of Pipistrellus sp. were retrieved from GenBank and added to the dataset. The obtained sequences were imported into the software Geneious Prime® (v. 2022.2.2 Biomatters Ltd.) where the alignment was performed using MAFFT v. 7.490 (Katoh et al., 2002; Katoh and Standley, 2013) under the default parameters. Phylogenetic analysis based on the cytb mitochondrial fragment was performed under a Bayesian inference (BI) method, using Hypsugo savii as the outgroup (following Mayer and Helversen, 2001). To determine the best-fitting nucleotide model, we used ModelFinder (Kalyaanamoorthy et al., 2017) from the IQ-TREE web server (Trifinopoulos et al., 2016). The software BEAST v. 2.6.6 (Bouckaert et al., 2019) was used for the BI topology. Analyses were run twice for 106 generations with a sampling frequency of 1000. Models and prior specifications applied were as follows (otherwise by default): strict clock, coalescent with constant population size, and the JC69 nucleotide model based on ModelFinder. Convergence for all model parameters was assessed by examining trace plots and histograms in Tracer v. 1.7.1 (Rambaut et al., 2018) after obtaining an effective sample size (ESS) greater than 200. The initial 10 % of samples were discarded as burn-in. Runs were combined using LogCombiner, and maximum credibility trees with divergence time means and 95 % highest probability densities (HPDs) were produced using TreeAnnotator. Trees were visualised using FigTree v. 1.4.4 (Rambaut, 2009). Calculation of genetic distances was performed using Mega v. 11 (Tamura et al., 2021) based on Kimura's two-parameter distance (Kimura, 1980).
2.6 Diet analyses
DNA was extracted from pellets collected from four out of the six captured Pipistrellus maderensis, using the E.Z.N.A Tissue DNA Kit (Omega Bio-Tek, Norcross, Georgia, USA). For this, we followed Mata et al. (2021), except that no Inhibitex tablets (Qiagen, Hilden, Germany) were used. DNA was then amplified by PCR using arthropod general cytochrome oxidase subunit 1 (COI) primers fwhF2-R2n (Vamos et al., 2017) modified with Illumina overhangs. Libraries were prepared following Mata et al. (2021) and finally sequenced in a MiSeq desktop sequencer (Illumina) using a MiSeq Reagent Kit v. 3 (2×250 bp). Bioinformatic processing of raw sequencing data was conducted using OBITOOLS (Boyer et al., 2016) and VSEARCH (Rognes et al., 2016) following Martins et al. (2022). Operational taxonomic units (OTUs) were taxonomically assigned using the Barcode of Life Data System (BOLD) public database under BOLDigger (Buchner and Leese, 2020).
During our bioacoustic survey we detected bats in 28 out of the 46 sampling sites (60 %; Table S2). In total, we identified 7797 bat passes of Pipistrellus maderensis in our recordings. Furthermore, we mist-netted six individuals (including three juveniles and one lactating female; Fig. S1; Table S4). All captures occurred at ca. 260 m a.s.l., in forest trails near Pico Castelo. No other bat species were detected.
3.1 Bat–environment relationships
Our GLMM results indicate that the activity of Pipistrellus maderensis is not strongly influenced by any of the considered land-use types (forest, agriculture, grassland, non-vegetated areas, built-up areas) (p>0.05; Table S5). However, the number of recorded bat passes increased with decreasing distance to water sources (p<0.001; Table S6 and Fig. 2a). GLMM residuals were not spatially autocorrelated for most models (P>0.05). However, for one of the GLMMs (non-vegetated areas), the test showed spatially structured residuals (P<0.05; Table S7).
3.2 Phylogenetic analyses
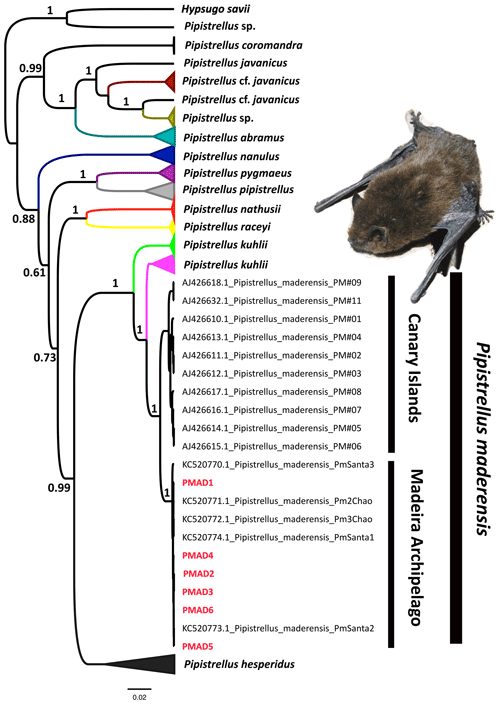
The obtained cytb fragment from the sequenced Pipistrellus sp. had a length of 779 bp. The Bayesian phylogenetic analysis clustered the bats sampled in Porto Santo with the specimens assigned as Pipistrellus maderensis, with high posterior probability (Fig. 3). Specifically, the genealogy suggests that these individuals have a close phylogenetic affinity with the specimens from Madeira Island, separated by 0.04 % of genetic distance. Moreover, the bats captured in Porto Santo share the exact same cytb haplotype, differing from the sequence KC520772.1 from Madeira by a single base pair (99.8 % of similarity among sequences). This highlights the low genetic diversity of the bat populations of the archipelago of Madeira, in comparison with the Canary Islands (98.7 % of identical nucleotide sites).
3.3 Diet analyses
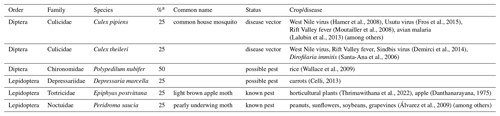
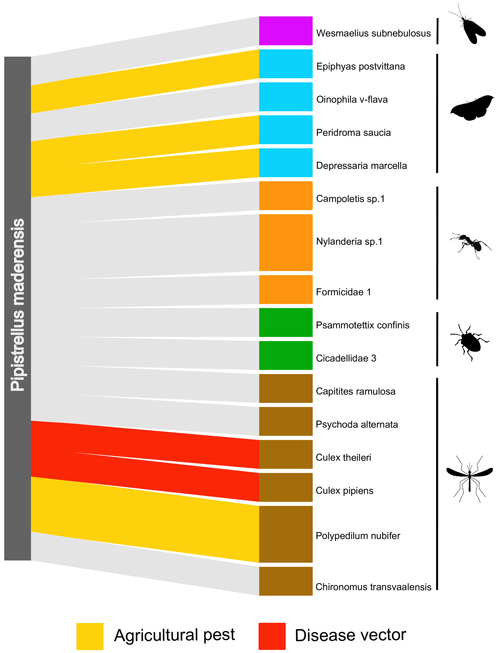
DNA material from the faecal samples collected from four individuals led to a total of 16 OTUs and 39 594 reads. In total, at least five orders, 12 families, 13 genera, and 11 species were identified to be consumed by Pipistrellus maderensis. Diptera was the predominant order detected, followed by Lepidoptera, Hymenoptera, Hemiptera, and lastly Neuroptera (Fig. 4). Of the identified prey, several are known agricultural insect pest species and disease vectors (Table 1).
Lack of baseline knowledge is a key constraint for the development of management strategies for the conservation of insectivorous bats and for maximising any ecosystem services they might provide. Here, we show that Porto Santo is still home to an extant population of Pipistrellus maderensis and provide much-needed information about their habitat affinities, phylogenetic kinship, and trophic interactions.
Bats are known to present marked responses to landscape features in both island and non-island ecosystems (Ancillotto et al., 2023; López-Baucells et al., 2021). In the nearby island of Madeira, Pipistrellus maderensis was found to be particularly associated with shrubland, agricultural areas, and Laurisilva (primary) forest (Ferreira et al., 2022). However, in Porto Santo and probably as an artefact of the small dimensions of the island, in which multiple land-use covers converge within relatively short distances, we were unable to detect these associations. Yet, consistent with previous studies (Ancillotto et al., 2019; Moretto et al., 2023; Torrent et al., 2018), we have found that bat activity was positively associated with artificial water reservoirs such as dams and urban ponds. Water limitations and unpredictability in precipitation, typical of xeric climates such as the one characterising Porto Santo (Maestre et al., 2016), pose important challenges to insectivorous bats (Conenna et al., 2021). Our results indicate that in Porto Santo artificial water sources likely provide important drinking and foraging resources for Pipistrellus maderensis, potentially reducing spatiotemporal variations in food availability. However, it is important to note that our bioacoustic survey was conducted over ca. 1 week – too short of a period to be able to capture complex bat activity variations, which can be affected by multiple factors, including lunar phase and season (Appel et al., 2021; Gorman et al., 2021).
The evolutionary history of insular species is often shaped by the geographic separation of different island populations (e.g. Recuerda et al., 2021). Previous molecular phylogenetic analyses suggested the existence of at least three lineages of Pipistrellus maderensis in the Canary Islands (Pestano et al., 2003) and one in Madeira (Jesus et al., 2013). Indeed, there is no haplotype sharing between Pipistrellus maderensis from both archipelagos, suggesting that they represent distinct evolutionary lineages, albeit not distinct enough to constitute different species (Jesus et al., 2013). Our results indicate a close phylogenetic affinity between the Pipistrellus maderensis from Porto Santo and Madeira (only 0.04 % genetic distance; Fig. 3), indicating that the ca. 40 km separating the two islands has not led to considerable genetic divergence between both populations.
Due to their capacity to fly, geographical barriers, such as large expanses of ocean, do not necessarily represent effective barriers to gene flow among bat populations (García-Mudarra et al., 2009). Yet, molecular studies revealed limited overwater dispersal of Nyctalus azoreum between some islands of the archipelago of the Azores (Salgueiro et al., 2008). Likewise, while dispersal and gene flow of Hypsugo savii among some of the closest islands (> 40 km) of the Canary Islands is frequent, gene flow is rare among Canarian populations of Pipistrellus maderensis inhabiting different islands (Pestano et al., 2003). The low genetic divergence between the populations of Pipistrellus maderensis of Porto Santo and Madeira suggests gene flow between both islands. However, this might also be due to a relatively recent (re)colonisation of Porto Santo, with individuals from Madeira. In fact, Porto Santo was heavily impacted by human-induced habitat change, which combined with the effects of invasive species might have led to the decline in the abundance – and possible extinction – of Pipistrellus maderensis and possibly other bat species, which would mimic patterns of anthropogenic extinctions observed in other vertebrate groups (e.g. Alcover et al., 2015).
DNA metabarcoding allows unprecedented resolution in the assessment of the diet of insectivorous predators. Although we were only able to sample and analyse faecal samples from four individuals, we detected prey items belonging to five insect orders, corresponding to at least 11 different species. In Madeira, a recent study identified Lepidoptera as the most consumed order by Pipistrellus maderensis (Gonçalves, 2022). However, Diptera was the most represented order in the four samples analysed from Porto Santo (Fig. 4). These results align with findings for Pipistrellus kuhlii (a sister taxa of P. maderensis) in the Iberian Peninsula, where Diptera has been found to make up over half of the species' diet (Goiti et al., 2003). Hymenoptera, Lepidoptera, Hemiptera, and Neuroptera were also detected in the diet of Pipistrellus maderensis in this study, which in combination with the identification of Coleoptera, Tipulidae, Chironomidae, and Aranea in the diet of the species in Madeira (Gonçalves, 2022) suggests that, similarly to Pipistrellus kuhlii (Amichai and Korine, 2020), Pipistrellus maderensis is likely a “selective opportunist” that feeds on a wide variety of prey, according to its availability. Despite the reduced sample size of our diet analyses, we detected at least four known or potential economically important insect pest species (e.g. Epiphyas postvittana, which is known to damage the fruits of multiple species; Danthanarayana et al., 1975; Thrimawithana et al., 2022) and two mosquitoes known to be vectors of human and non-human diseases (e.g. Culex pipiens, which can spread avian malaria; Hamer et al., 2008; Fros et al., 2015) (Table 1). The high percentage of known or potential agricultural pest species and of species of health relevance consumed by Pipistrellus maderensis highlights the potential role of insectivorous bats as suppressors of arthropods with negative economic and (both human and non-human) disease implications (Montauban et al., 2021; Ancillotto et al., 2022, 2023; Ferreira et al., 2023).
Insectivorous bats are often not considered as charismatic as other island vertebrates, and their conservation is neglected. The population of Pipistrellus maderensis from Porto Santo, despite being genetically similar to the one inhabiting Madeira, is of critical importance to the ecological balance, acting as a predator of a multitude of arthropod species. The bats were detected throughout most of the island, but the extant population appears to be small and thus particularly vulnerable to threat factors such as the destruction of roost and feeding habitats, reduction of prey due to pesticide use, or the impacts of invasive species (e.g. free-ranging cats Felis catus, a known predator of Pipistrellus maderensis; Rocha, 2015). We urge that more research is devoted to this population, which represents the sole native terrestrial mammal of Porto Santo.
All DNA sequences are available on GenBank with accession numbers OQ260001 to OQ260006.
The supplement related to this article is available online at: https://doi.org/10.5194/we-23-87-2023-supplement.
Conceptualisation: RR and EKN. Methodology: RR, EKN, NT, AG, AR, EJS, SPR, VAM, and CR. Writing – original draft preparation: RR and EKN. Writing – review and editing: NT, AG, AR, EJS, SPR, VAM, and CR. Project administration: RR. Funding acquisition: RR and CR. All authors have approved the submitted version of the manuscript.
At least one of the (co-)authors is a member of the editorial board of Web Ecology. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors would like to thank Inês Órfão and Luísa Drumond for assisting during fieldwork and Hugo Rebelo for general support. This research was conducted under permit 10/IFCN/2019 provided by the Institute of Forests and Nature Conservation from the Autonomous Region of Madeira. The authors acknowledge Rym Nouioua's support on the acoustic analysis and Diogo Ferreira's support on the statistical analysis.
This research has been supported by the National Geographic Society (grant no. EC-64368R-20) and the European Regional Development Fund (ERDF) (grant no. PTDC/BIA-EVL/27958/2017–POCI-01-0145-FEDER-027958; CR). Vanessa A. Mata and Ricardo Rocha were supported by the Fundação para a Ciência e a Tecnologia (FCT) through the programme “Stimulus of Scientific Employment, Individual Support – 3rd Edition” (contract nos. 2020.02547.CEECIND and 2020.01129.CEECIND, respectively). Ricardo Rocha was further supported by a postdoctoral fellowship from ARDITI – Madeira's Regional Agency for the Development of Research, Technology and Innovation (grant no. M1420-09-5369-FSE-000002). Catarina Rato was supported by a FCT postdoctoral contract (grant no. DL57/2016/CP1440/CT0005), and Nia Toshkova was supported by the Bulgarian Academy of Sciences via the Erasmus+ programme.
This paper was edited by Daniel Montesinos and reviewed by M. Brock Fenton and three anonymous referees.
Alcalde, J. and Juste, J.: Pipistrellus maderensis (Internet; cited 10 June 2019), The IUCN red list of threatened species, in: IUCN Red List of Threatened Species, RLTS.T17315A1380378.en, https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T17315A1380378.en, 2016.
Alcover, J. A., Pieper, H., Pereira, F., and Rando, J. C.: Five new extinct species of rails (Aves: Gruiformes: Rallidae) from the Macaronesian Islands (North Atlantic Ocean), Zootaxa, 4057, 151–190, https://doi.org/10.11646/zootaxa.4057.2.1, 2015.
Álvarez, A., Virla, E. G., Pera, L. M., and Baigorí, M. D.: Characterization of native Bacillus thuringiensis strains and selection of an isolate active against Spodoptera frugiperda and Peridroma saucia, Biotechnol. Lett., 31, 1899–1903, https://doi.org/10.1007/s10529-009-0091-5, 2009.
Amichai, E. and Korine, C.: Kuhl's Pipistrelle Pipistrellus kuhlii (Kuhl, 1817), in: Handbook of the Mammals of Europe, edited by: Hackländer, K. and Zachos, F. E., Handbook of the Mammals of Europe, Springer, Cham. https://doi.org/10.1007/978-3-319-65038-8_69-1, 2020.
Ancillotto, L., Bosso, L., Salinas-Ramos, V. B., and Russo, D.: The importance of ponds for the conservation of bats in urban landscapes, Landscape Urban Plan., 190, 103607, https://doi.org/10.1016/j.landurbplan.2019.103607, 2019.
Ancillotto, L., Rummo, R., Agostinetto, G., Tommasi, N., Garonna, A. P., de Benedetta, F., Bernardo, U., Galimberti, A., and Russo, D.: Bats as suppressors of agroforestry pests in beech forests, Forest Ecol. Manage., 522, 120467, https://doi.org/10.1016/j.foreco.2022.120467, 2022.
Ancillotto, L., Scaramella, C., Dartora, F., Migliozzi, A., and Russo, D.: Organic farming sustains bats in Mediterranean farmland, Agr. Ecosyst. Environ., 342, 108230, https://doi.org/10.1016/j.agee.2022.108230, 2023.
Appel, G., López-Baucells, A., Rocha, R., Meyer, C. F., and Bobrowiec, P. E. D.: Habitat disturbance trumps moonlight effects on the activity of tropical insectivorous bats, Anim. Conserv., 24, 1046–1058, 2021.
Bartón, K.: MuMIn: multi-model inference, R package version 1.9.13, Vol. 43, p. 6, 2020.
Blackburn, T. M., Cassey, P., Duncan, R. P., Evans, K. L., and Gaston, K. J.: Avian extinction and mammalian introductions on oceanic islands, Science, 305, 1955–1958, https://doi.org/10.1126/science.1101617, 2004.
Borges, P. A. V., Abreu, C., Aguiar, A. F., Carvalho, P., Fontinha, S., Jardim, R., Melo, I., Oliveira, P., Sequeira, M. M., Sérgio, C., Serrano, A. R., Sim-Sim, M., and Vieira, P.: Terrestrial and freshwater biodiversity of the Madeira and Selvagens archipelagos, in: A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos, edited by: Borges, P. A. V., Abreu, C., Aguiar, A. F., Carvalho, P., Fontinha, S., Jardim, R., Melo, I., Oliveira, P., Sequeira, M. M., Sérgio, C., Serrano, A. R., and Vieira, P., Direcção Regional do Ambiente da Madeira e Universidade dos Açores, Açores, 13–25, http://hdl.handle.net/10400.3/1955 (last access: January 2022), 2008.
Bouckaert, R., Vaughan, T. G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., and De Maio, N.: BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis, PLoS Comput. Biol., 15, e1006650, https://doi.org/10.1371/journal.pcbi.1006650, 2019.
Boyer, F., Mercier, C., Bonin, A., Le Bras, Y., Taberlet, P., and Coissac, E.: obitools: A unix-inspired software package for DNA metabarcoding, Molecular Ecol. Resour., 16, 176–182, 2016.
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., Skaug, H. J., Maechler, M., and Bolker, B. M.: glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling, The R Journal, 9, 378–400, 2017.
Buchner, D. and Leese, F.: BOLDigger – a Python package to identify and organise sequences with the Barcode of Life Data systems, Metabarcod. Metagenom., 4, 19–21. https://doi.org/10.3897/mbmg.4.53535, 2020.
Burnham, K. P. and Anderson, D. R.: Model selection and multimodel inference: a practical information-theoretic approach, 2nd Edn., New York, Springer-Verlag, 2002.
Cabral, M. J., Almeida, J., Almeida, P. R., Dellinger, T., Ferrand de Almeida, N., Oliveira, M. E., and Santos-Reis, M.: Livro vermelho dos vertebrados de Portugal, Instituto de Conservação da Natureza, Lisbon, Portugal, 2005.
Carvalho, M. and Osborne, J.: Vicia ferreirensis. The IUCN red list of threatened species 2011, https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T180553A7646823.en, 2011.
Celli, G.: Studies on a lepidopterous pest, Depressaria marcella Rebel (Lepidoptera: Oecophoridae), injurious to the seed carrot crops (Daucus carota L.) and research for a rational control. Bollettino dell'Istituto di Entomologio della Universita degli Studi di Bologna, 29, 1–47, 2013.
Conenna, I., Rocha, R., Russo, D., and Cabeza, M.: Insular bats and research effort: a review of global patterns and priorities, Mammal Rev., 47, 169–182, 2017.
Conenna, I., Santini, L., Rocha, R., Monadjem, A., Cabeza, M., and Russo, D.: Global patterns of functional trait variation along aridity gradients in bats, Global Ecol. Biogeogr., 30, 1014–1029. https://doi.org/10.1111/geb.13278, 2021
Danthanarayana, W.: The bionomics, distribution and host range of the light brown apple moth, Epiphyas postvittana (Walk.) (Tortricidae), Aust. J. Zool., 23, 309–319, https://doi.org/10.1071/ZO9750419, 1975.
Demirci, B., Durmaz, E., and Alten, B.: Influence of bloodmeal source on reproductive output of the potential West Nile Vector, Culex theileri (Diptera: Culicidae), J. Med. Entomol., 51, 1312–1316. https://doi.org/10.1603/ME13197, 2014.
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., Marquéz, J. R. G., Gruber, B., Lafourcade, B., Leitao, P. J., and Münkemüller, T.: Collinearity: a review of methods to deal with it and a simulation study evaluating their performance, Ecography, 36, 27–46, 2013.
Emerson, B. C.: Evolution on oceanic islands: molecular phylogenetic approaches to understanding pattern and process, Mol. Ecol., 11, 951-966, https://doi.org/10.1146/annurev.publhealth.23.100901.140546, 2002.
Fernández-Palacios, J. M., Kreft, H., Irl, S. D. H., Norder, S., Ah-Peng, C., Borges, P. A. V., Burns, K. C., de Nascimento, L., Meyer, J. Y., Montes, E., and Drake, D. R.: Scientists' warning - The outstanding biodiversity of islands is in peril, Global Ecol. Conserv., 31, e01847, https://doi.org/10.1016/j.gecco.2021.e01847, 2021.
Ferreira, D. F., Gibb, R., López-Baucells, A., Nunes, N. J., Jones, K. E., and Rocha, R.: Species-specific responses to land-use change in island insectivorous bats, J. Nat. Conserv., 67, 126177, https://doi.org/10.1016/j.jnc.2022.126177, 2022
Ferreira, D. F., Jarrett, C., Wandji, A. C., Atagana, P. J., Rebelo, H., Maas, B., and Powell, L. L.: Birds and bats enhance yields in Afrotropical cacao agroforests only under high tree-level shade cover, Agr. Ecosyst. Environ., 345, 108325, https://doi.org/10.1016/j.agee.2022.108325, 2023.
Frick, W. F., Kingston, T., and Flanders, J.: A review of the major threats and challenges to global bat conservation, Ann. NY Acad. Sci., 1469, 5–25, https://doi.org/10.1111/nyas.14045, 2020.
Fros, J. J., Miesen, P., Vogels, C. B., Gaibani, P., Sambri, V., Martina, B. E., Koenraadt, C. J., van Rij, R. P., Vlak, J. M., Takken, W., and Pijlman, G. P.: Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe, One Health, 1, 31–36, https://doi.org/10.1016/j.onehlt.2015.08.002, 2015.
García-Mudarra, J. L., Ibáñez, C., and Juste, J.: The straits of Gibraltar: barrier or bridge to Ibero-Moroccan bat diversity?, Biol. J. Linn. Soc., 96, 434–450, https://doi.org/10.1111/j.1095-8312.2008.01128.x, 2009.
Goiti, U., Vecin, P., Garin, I., Saloña, M., and Aihartza, J. R.: Diet and prey selection in Kuhl's pipistrelle Pipistrellus kuhlii (Chiroptera: Vespertilionidae) in south-western Europe, Acta Theriol (Warsz), 48, 457–468, https://doi.org/10.1007/BF03192492, 2003.
Golzáles-Dionis, J., Ruiz, C. C., Cruzado-Caballero, P., Cadavid-Melero, E., and Crespo, V. D.: First study of the bat fossil record of the mid-Atlantic volcanic islands, Earth Environ. Sci. T. R. So., 113, 13–27, https://doi.org/10.1017/S1755691021000384, 2022.
Gonçalves, A.: A metabarcoding assessment of the diet of insectivorous bats in a subtropical oceanic island. MSc thesis, Faculty of Sciences of the University of Porto, Department of Biology, 52 pp., https://hdl.handle.net/10216/146885, 2022.
Gorman, K. M., Barr, E. L., Ries, L., Nocera, T., and Ford, W. M.: Bat activity patterns relative to temporal and weather effects in a temperate coastal environment, Global Ecol. Conserv., 30, p.e01769, https://doi.org/10.1016/j.gecco.2021.e01769, 2021.
Hamer, G. L., Kitron, U. D., Brawn, J. D., Loss, S. R., Ruiz, M. O., Goldberg, T. L., and Walker, E. D.: Culex pipiens (Diptera: Culicidae): A bridge vector of West Nile virus to humans, Jo. Med. Entomol., 45, 125–128, https://doi.org/10.1603/0022-2585(2008)45[125:CPDCAB]2.0.CO;2, 2008.
Hill, A. P., Prince, P., Piña Covarrubias, E., Doncaster, C. P., Snaddon, J. L., and Rogers, A.: AudioMoth: Evaluation of a smart open acoustic device for monitoring biodiversity and the environment, Methods Ecol. Evol., 9, 1199–1211, https://doi.org/10.1111/2041-210X.12955, 2018
Ibáñez, C., García-Mudarra, J. L., Ruedi, M., Stadelmann, B., and Juste, J.: The Iberian contribution to cryptic diversity in European bats, Acta Chiropterol., 8, 277–297, https://doi.org/10.3161/150811006779398582, 2006.
Jesus, J., Teixeira, S., Teixeira, D., Freitas, T., and Russo, D.: Vertebrados Terrestres Autóctones dos Arquipélagos da Madeira e Selvagens – Répteis e Mamíferos, Colecção Biodiversidade Madeirense: Avaliação e Conservação no 6, 118, ISBN 978-989-95790-4-0, 2009.
Jesus, J., Teixeira, S., Freitas, T., Teixeira, D., and Brehm, A.: Genetic identity of Pipistrellus maderensis from the madeira archipelago: A first assessment, and implications for conservation, Hystrix, 24, 2, https://doi.org/10.4404/hystrix-24.2-8736, 2013.
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., and Jermiin, L. S.: ModelFinder: fast model selection for accurate phylogenetic estimates, Nat. Methods, 14, 587–589, https://doi.org/10.1038/nmeth.4285, 2017.
Katoh, K. and Standley, D. M.: MAFFT multiple sequence alignment software version 7: improvements in performance and usability, Mol. Biol. Evol., 30, 772–780, https://doi.org/10.1093/molbev/mst010, 2013.
Katoh, K., Misawa, K., Kumar, K., and Miyata, T.: MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform, Nucleic Acids Res., 30, 3059–3066, https://doi.org/10.1093/nar/gkf436, 2002.
Kemp, J., López-Baucells, A., Rocha, R., Wangensteen, O. S., Andriatafika, Z., Nair, A., and Cabeza, M.: Bats as potential suppressors of multiple agricultural pests: A case study from Madagascar, Agr. Ecosyst. Environ., 269, 88–96, https://doi.org/10.1016/j.agee.2018.09.027, 2019.
Kier, G., Kreft, H., Tien, M. L., Jetz, W., Ibisch, P. L., Nowicki, C., Mutke, J., and Barthlott, W.: A global assessment of endemism and species richness across island and mainland regions, P. Natl. Acad. Sci. USA, 106, 9322–9327, https://doi.org/10.1073/pnas.0810306106, 2009.
Kimura, M.: A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences, J. Mol. Evol., 16, 111–20, https://doi.org/10.1007/BF01731581, 1980.
Kratochwil, A. and Schwabe, A.: Wild bees (Anthophila) of Porto Santo (Madeira Archipelago) and their habitats: Species diversity, distribution patterns and bee-plant network, Linzer biol. Beitr, 50, 1219–1247, 2018.
Lalubin, F., Delédevant, A., Glaizot, O., and Christe, P.: Temporal changes in mosquito abundance (Culex pipiens), avian malaria prevalence and lineage composition, Parasites Vect., 6, 1–8, https://doi.org/10.1186/1756-3305-6-307, 2013.
Leclerc, C., Courchamp, F., and Bellard, C.: Insular threat associations within taxa worldwide, Sci. Rep., 8, 6393, https://doi.org/10.1038/s41598-018-24733-0, 2018
López-Baucells, A., Flaquer, C., Mas, M., Pons, P., and Puig-Montserrat, X.: Recurring fires in Mediterranean habitats and their impact on bats, Biodiv. Conserv., 30, 385–402, 2021.
López-Bosch, D., Rocha, R., López-Baucells, A., Wang, Y., Si, X., Ding, P., Gibson, L., and Palmeirim, A. F.: Passive acoustic monitoring reveals the role of habitat affinity in sensitivity of sub-tropical East Asian bats to fragmentation, Remote Sens. Ecol. Conserv., 8, 208–221, https://doi.org/10.1002/rse2.237, 2022.
Maestre, F. T., Eldridge, D. J., Soliveres, S., Kéfi, S., Delgado-Baquerizo, M., Bowker, M. A., and Berdugo, M.: Structure and functioning of dryland ecosystems in a changing world, Annu. Rev. Ecol. Evol. S., 47, 215–237, 2016.
Martins, B., Silva-rocha, I., Mata, V. A., Gonçalves, Y., Rocha, R., and Rato, C.: Trophic interactions of an invasive gecko in an endemic-rich oceanic island: Insights using DNA metabarcoding, Front. Ecol. Evol., 10, 1044230, https://doi.org/10.3389/fevo.2022.1044230, 2022.
Mata, V. A., da Silva, L. P., Veríssimo, J., Horta, P., Raposeira, H., McCracken, G. F., Rebelo, H., and Beja, P.: Combining DNA metabarcoding and ecological networks to inform conservation biocontrol by small vertebrate predators, Ecol. Appl., 31, 1–15, https://doi.org/10.1002/eap.2457, 2021.
Matthews, T. J., Wayman, J. P., Cardoso, P., Sayol, F., Hume, J. P., Ulrich, W., Tobias, J. A., Soares, F. C., Thébaud, C., Martin, T. E., and Triantis, K. A.: Threatened and extinct island endemic birds of the world: Distribution, threats and functional diversity, J. Biogeogr., 49, 1920–1940, https://doi.org/10.1111/jbi.14474, 2022.
Mayer, F. and Helversen, Ov.: Cryptic diversity in European bats, P. Roy. Soc. B-Biol. Sci., 268, 1825–1832, https://doi.org/10.1098/rspb.2001.1744, 2001.
Montauban, C., Mas, M., Wangensteen, O. S., Sarto i Monteys, V., Fornós, D. G., Mola, X. F., and López-Baucells, A.: Bats as natural samplers: First record of the invasive pest rice water weevil Lissorhoptrus oryzophilus in the Iberian Peninsula, Crop. Protect. 141, 105427, https://doi.org/10.1016/j.cropro.2020.105427, 2021.
Moretto, L., Ancillotto, L., Li, H., Threlfall, C. G., Jung, K., and Avila-Flores, R.: City Trees, Parks, and Ponds: Green and Blue Spaces as Life Supports to Urban Bats, in: Urban Bats, edited by: Moretto, L., Coleman, J. L., Davy, C. M., Fenton, M. B., Korine, C., and Patriquin, K. J., Springer, Cham, 107–121, https://doi.org/10.1007/978-3-031-13173-8_8, 2023.
Moutailler, S., Krida, G., Schaffner, F., Vazeille, M., and Failloux, A. B.: Potential vectors of rift valley fever virus in the Mediterranean region, Vector-Borne Zoonot., 8, 749–753, https://doi.org/10.1089/vbz.2008.0009, 2008.
Nogué, S., Santos, A. M. C., Birks, H. J. B., Björck, S., Castilla-Beltrán, A., Connor, S., Boer E. J., Nascimento, L., Felde, V. A., Fernández-Palacios, J. M., Froyd, C. A., Haberle, S. G., Hooghiemstra, H., Ljung, K., Norder, S. J., Peñuelas, J., Prebble, M., Stevenson, J., Whittaker, R. J., Willis, K. J., Wilmshurst, J. M., and Steinbauer, M. J.: The human dimension of biodiversity changes on islands, Science, 372, 488–491, https://doi.org/10.1126/science.abd6706, 2021.
Nori, J., Villalobos, F., Osorio-Olvera, L., and Loyola, R.: Insufficient protection and intense human pressure threaten islands worldwide, Perspect. Ecol. Conserv., 20, 223–230, https://doi.org/10.1016/j.pecon.2022.06.003, 2022.
Nouioua, R.: Effects of large-scale forest fires on insular bats, MSc thesis, University of Bremen, 1–38, 2022.
Pestano, J., Brown, R. P., Suárez, N. M., and Fajardo, S.: Phylogeography of pipistrelle-like bats within the Canary Islands, based on mtDNA sequences, Mol. Phylogenet. Evol., 26, 56–63, 2003.
Queiroz A., Alves P., Barroso I., Beja P., Fernandes M., Freitas L., Mathias M., Mira A., Palmeirim J. M., Prieto R., Rainho A., Rodrigues L., Santos-Reis M., and Sequeira M.: Pipistrellus maderensis, in: Livro vermelho dos vertebrados de Portugal, Instituto da Conservação da Natureza, Lisboa, edited by: Cabral, M., Almeida, J., Almeida, P., Dellinger, T., Ferrand de Almeida, N., Oliveira, M., Palmeirim, J., Queiroz, A., Rogado, L., and Santos-Reis, M., 457–458, 2005.
Rainho, A.: Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal), Diversity, 14, p. 17, https://doi.org/10.3390/d14010017, 2022.
Rainho, A., Marques J. T., and Palmeirim, J.: Os Morcegos dos Arquipélagos dos Açores e da Madeira: Um contributo para a sua conservação, Lisboa: Centro de Biologia Ambiental, Instituto de Conservação de Natureza, https://doi.org/10.13140/RG.2.1.4314.0960, 2002.
Rambaut, A.: Figtree [Computer Software], http://tree.bio.ed.ac.uk/software/figtree/ (last access: January 2023), 2009.
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., and Suchard, M. A.: Posterior summarisation in Bayesian phylogenetics using Tracer 1.7, Syst. Biol., syy032, https://doi.org/10.1093/sysbio/syy032, 2018.
Ramírez-Fráncel, L. A., García-Herrera, L. V., Losada-Prado, S., Reinoso-Flórez, G., Sánchez-Hernández, A., Estrada-Villegas, S., and Guevara, G.: Bats and their vital ecosystem services: a global review, Integr. Zool., 17, 2–23, https://doi.org/10.1111/1749-4877.12552, 2021.
Recuerda, M., Illera, J. C., Blanco, G., Zardoya, R., and Milá, B.: Sequential colonization of oceanic archipelagos led to a species-level radiation in the common chaffinch complex (Aves: Fringilla coelebs), Mol. Phylogenet. Evol., 164, 197291, https://doi.org/10.1016/j.ympev.2021.107291, 2021.
Rivas-Martínez, S.: Ensayo geobotánico global sobre la Macaronesia, in: Homenaje al Prof. Wolfredo Wildpret de la Torre, edited by: Beltrán, E., Tejera, J., Alfonso-Carrilo, A., Garcia Gallo, and Rodríguez Delgado, O., Inst. Est. Canarios, La Laguna, Tenerife, 255–296, 2009.
Rocha, R.: Look what the cat dragged in: Felis silvestris catus as predators of insular bats and instance of predation on the endangered Pipistrellus maderensis, Barbastella, 8, 8–11, https://doi.org/10.14709/barbj.8.1.2015.04, 2015.
Rocha, R.: Madeiran pipistrelle Pipistrellus maderensis (Dobson, 1878), Handbook of the Mammals of Europe, 1–9, https://doi.org/10.1007/978-3-319-65038-8_70-1, 2021.
Rocha, R., Sequeira, M. M., Douglas, L. R., Gouveia, M., Jardim, R., Jesus, J., Jones, H. P., and Russo, D.: Extinctions of introduced game species on oceanic islands: curse for hunters or conservation opportunities?, Biodiv. Conserv., 26, 2517–2520, https://doi.org/10.1007/s10531-017-1363-3, 2017.
Rodríguez-Durán, A., Pérez, J., Montalbán, M. A., and Sandoval, J. M.: Predation by free-roaming cats on an insular population of bats. Acta Chiropterol., 12, 359–362, https://doi.org/10.3161/150811010X537945, 2010.
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahé, F.: VSEARCH: A versatile open source tool for metagenomics, PeerJ, 2016, 1–22, https://doi.org/10.7717/peerj.2584, 2016.
Russell, J. C. and Kueffer, C.: Island Biodiversity in the Anthropocene, Ann. Rev. Environ. Res., 44, 31–60, https://doi.org/10.1146/annurev-environ-101718-033245, 2019.
Salgueiro, P., Palmeirim, J. M., Ruedi, M. et al.: Gene flow and population structure of the endemic Azorean bat (Nyctalus azoreum) based on microsatellites: implications for conservation, Conserv. Genet., 9, 1163–1171, https://doi.org/10.1007/s10592-007-9430-z, 2008.
Santa-Ana, M., Khadem, M., and Capela, R.: Natural infection of Culex theileri (Diptera: Culicidae) with Dirofilaria immitis (Nematoda: Filarioidea) on Madeira Island, Portugal, J. Med. Entomol., 43, 104–106, https://doi.org/10.1093/jmedent/43.1.104, 2006.
Sikes, R. S., Gannon, W. L., and Care, A.: Mammalogists, U.C.o.t.A.S.o.: Guidelines of the American Society of Mammalogists for the use of wild mammals in research, J. Mammal., 92, 235–253, https://doi.org/10.1644/10-MAMM-F-355.1, 2011.
Smith, M. F. and Patton, J. L.: The diversification of South American murid rodents: evidence from mitochondrial DNA sequence data for the akodontine tribe, Biol. J. Linn. Soc., 50, 149–177, https://doi.org/10.1111/j.1095-8312.1993.tb00924.x, 1993.
Sparrius, L. B., Aptroot, A., Sipman, H. J. M., Pérez-Vargas, I., Matos, P., Gerlach, A., and Vervoort, M.: Estimating the population size of the endemic lichens Anzia centrifuga (Parmeliaceae) and Ramalina species (Ramalinaceae) on Porto Santo (Madeira archipelago), Bryologist, 120, 293–301, https://doi.org/10.1639/0007-2745-120.3.293, 2017.
Tamura, K., Stecher, G., and Kumar, S.: MEGA11: Molecular Evolutionary Genetics Analysis version 11, Mol. Biol. Evolut., 38, 3022–3027, https://doi.org/10.1093/molbev/msab120, 2021.
Teixeira, S. and Jesus, J.: Echolocation calls of bats from Madeira Island: acoustic characterization and implications for surveys, Acta Chiropterol., 11, 183–190, https://doi.org/10.3161/150811009X465802, 2009.
Thrimawithana, A. H., Wu, C., Christeller, J. T., Simpson, R. M., Hilario, E., Tooman, L. K., Begum, D., Jordan, M. D., Crowhurst, R., Newcomb, R. D., and Grapputo, A.: The Genomics and Population Genomics of the Light Brown Apple Moth, Epiphyas postvittana, an Invasive Tortricid Pest of Horticulture, Insects, 13, 264, https://doi.org/10.3390/insects13030264, 2022.
Torrent, L., López-Baucells, A., Rocha, R., Bobrowiec, P. E., and Meyer, C. F.: The importance of lakes for bat conservation in Amazonian rainforests: an assessment using autonomous recorders, Remote Sens. Ecol. Conserv., 4, 339–351, 2018.
Trifinopoulos, J., Nguyen, L.-T., von Haeseler, A., and Minh, B. Q.: W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis, Nucleic Acids Res., 44, W232–W235, https://doi.org/10.1093/nar/gkw256, 2016.
Trujillo, D. and Gonzalez, C.: Pipistrellus maderensis (Dobson, 1878) (Chiroptera: Vespertilionidae), una nueva adicion a la fauna de las islas Azores (oceano Atlantico), Vieraea , 39, 215–218, 2011.
Vamos, E. E., Elbrecht, V., and Leese, F.: Short coi markers for freshwater macroinvertebrate metabarcoding, Metabarcod. Metagenom., 1, 1–20, https://doi.org/10.3897/mbmg.1.14625, 2017.
Wallace, G. S., Mabee, W. R., and Combes, M. D.: Range Extension of a Nonindigenous Midge, Polypedilum nubifer (Diptera: Chironomidae), in North America, Southeastern Natural., 8, 559–562, https://doi.org/10.1656/058.008.0319, 2009.
Woinarski, J.: A Bat's End: The Christmas Island Pipistrelle and Extinction in Australia, Clayton South, CSIRO Publishing, ISBN 9781486308644, 2018.
Yoh, N., Clarke, J. A., López-Baucells, A., Mas, M., Bobrowiec, P. E., Rocha, R., and Meyer, C. F.: Edge effects and vertical stratification of aerial insectivorous bats across the interface of primary-secondary Amazonian rainforest, PLoS One, 17, p.e0274637, https://doi.org/10.1038/s41564-021-00880-5, 2022.