the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Invasive shallow-water foraminifera impacts local biodiversity mostly at densities above 20 %: the case of Corfu Island
Anna E. Weinmann
Olga Koukousioura
Maria V. Triantaphyllou
Martin R. Langer
Corfu Island (Greece) is located in the northern Ionian Sea and exhibits unique and diverse marine coastal habitats suitable for high-diversity assemblages such as shallow-water foraminifera. The island also lies near the current range expansion front of the invasive species Amphistegina lobifera. We analyzed the foraminiferal assemblages of 51 samples from 25 sites around the island, calculated diversity indices, and analyzed the community structures of foraminiferal assemblages in comparison to local environmental variables. In addition to that, using the spatial structure or relative abundances, we evaluated the effect of A. lobifera on the species richness of all benthic foraminifera and habitat-specific groups.
With 200 benthic foraminiferal species found, the high species richness and other diversity indices indicate Corfu as an area of high diversity. The main ecological drivers for the assemblage compositions were water depth, sediment texture, and habitat (especially vegetation), resulting in three main assemblage clusters around the island: (1) sandy or rocky, shallow-water areas from the south and west; (2) deeper areas from the west; and (3) rocky, vegetated areas of variable depths from the northwest and northeastern parts of the island.
Our analyses suggest that the invasive species A. lobifera affects local diversity of the foraminiferal assemblage and that these effects become apparent when the invasive species accounts for more than 10 %–20 % of the total abundance. We also observed significant negative correlations with sessile epiphytes and smaller miliolids. Both groups share similar microhabitats with A. lobifera and might be outcompeted, which is probably further facilitated by ongoing ocean warming. However, other warm-affiliated taxa (e.g., other symbiont-bearing species) initially show a positive correlation with the increasing presence of A. lobifera until the latter exceeds 20 %. We expect that A. lobifera and other warm-adapted species will play an increasing role in shaping future biodiversity and assemblage composition in this area, a feature that supports the prognosed tropicalization of the Mediterranean Sea.
- Article
(4426 KB) - Full-text XML
- BibTeX
- EndNote
Shallow-water foraminiferal assemblages are ubiquitous in marine nearshore environments and can be used as indicators for environmental conditions and quality status, which is widely applied in the Mediterranean Sea (e.g., Barras et al., 2014; Dimiza et al., 2016a, b; Parent et al., 2021). Previous analyses of foraminifera on Corfu Island were performed on fossil material from the Neogene and Pleistocene (Maragoudakis, 1961, 1967; Triantaphyllou et al., 1995; Rögl et al., 1997). For the Pleistocene, these studies report diverse foraminiferal assemblages dominated by rotaliids, small miliolids, and rare occurrences of peneroplids (Maragoudakis, 1967; Rögl et al., 1997). Langer and Mouanga (2016) included three recent samples from Corfu (Agnos and Boukaris) in their analysis on the range expansion of the non-indigenous Amphistegina lobifera Larsen (1976) into the Adriatic Sea. Yet, to date and to our knowledge, there is no comprehensive analysis of recent benthic shallow-water foraminifera from environments along the Corfu coast.
Corfu Island lies in the northern central Mediterranean and in the transition zone between the Ionian and Adriatic seas. The central Mediterranean used to include a biogeographic barrier between the western and eastern Mediterranean basins, which was attributed to the position of the 15 ∘C winter isotherm (e.g., Bianchi, 2007). This thermal barrier prevented extensive faunal exchange between the cooler western and warmer eastern basin (Coll et al., 2010; Bianchi et al., 2012; Langer et al., 2012). It also caused the isolation of the warm eastern basin from the tropical Atlantic (Albano et al., 2021). However, with ongoing climate warming (Pisano et al., 2020) and the northward shift of the thermal barrier (Bianchi et al., 2012), faunal exchange and overlapping biotas have become more frequent (e.g., Di Lorenzo et al., 2018; Massi et al., 2021). The extensive range expansions of tropical taxa into the central and even western Mediterranean also include benthic foraminifera, and an important example is the non-indigenous Amphistegina lobifera (Langer et al., 2012; Weinmann et al., 2013; Guastella et al., 2019, 2021). This species is assumed to have entered the eastern Mediterranean via the Suez Canal and has since expanded its distribution range into the central Mediterranean (Langer et al., 2012, and references therein; Guastella et al., 2019). In addition to its range expansion, it has been classified as a prioritized species in the sense of EU Regulation 1143/2014 on invasive alien species (Stulpinaite et al., 2020; Tsiamis et al., 2020). In some areas, it has already become so abundant that its empty shells form sediment layers of up to 50 cm thickness (Yokeş et al., 2014). The current range expansion of A. lobifera is further facilitated by ongoing ocean warming (Langer et al., 2012; Weinmann et al., 2013; Langer and Mouanga, 2016; Guastella et al., 2019, 2021).
Amphistegina lobifera affects the local foraminiferal fauna and as an ecosystem engineer shapes local habitats (e.g., transitions from rocky coasts to sandy environments; see discussion in Langer and Mouanga, 2016). Current range limits include the southern Adriatic Sea (Albania; Langer and Mouanga, 2016), the islands of Pantelleria and Favignana (Guastella et al., 2019), and Tunisia (southern coast; El Kateb et al., 2018; northern coast; Martin R. Langer, unpublished record), but, so far, there are no records from the Italian mainland (Mouanga, 2018; Stulpinaite et al., 2020). With southern Albania being the only known site of presence in the Adriatic Sea to date, Corfu Island is still situated near the northern front of the distribution range. The exact timing of the arrival of A. lobifera is unknown. Its first documented occurrence in Corfu is from material that was collected in 2010 (Langer et al., 2012; Langer and Mouanga, 2016). In the 2000s and 2010s, there are several records from other Ionian islands and the Albanian mainland: Kefalonia in 2002 (NHMW collection, unpublished data), Ithaka and Lefkada in 2012 (Mouanga and Langer, 2014), Zakynthos in 2012 (Triantaphyllou and Dimiza, 2013), and southern Albania in 2014 (Langer and Mouanga, 2016). Significant numbers of A. lobifera in these locations (up to 21 % in Ithaka, 75 % in Zakynthos, and 75 % in Albania) suggest an earlier arrival of this taxon in the respective areas, to form viable and thriving populations. This was also reported from Malta, where earliest records of A. lobifera from core sediments date back to the 1940s (Guastella et al., 2021). The same authors showed that the abundance of A. lobifera has been significantly increasing since the early 1990s (Guastella et al., 2021), with the first official recording of its presence dating from 2006 (Yokeş et al., 2007).
With the good ecological status of Corfu Island and moderate anthropogenic influence (no major industry apart from tourism), we expect a well-developed and pristine community of shallow-water foraminifera which is not overly stressed and can work as a good baseline for an assemblage structure under the effect of the invasive species Amphistegina lobifera. Therefore, the main objectives of the study are to (1) provide the first comprehensive faunal inventory of recent shallow-water foraminifera from Corfu, (2) analyze the spatial distribution and diversity of foraminiferal assemblages with regard to local environmental and ecological features, and (3) evaluate the effects of the establishment of the invasive species Amphistegina lobifera on the diversity and structure of the shallow-water benthic foraminiferal assemblages.
2.1 Study area
With an area of around 610 km2, Corfu is the second-largest island of the Ionian archipelago. It is part of the external Hellenides Ionian geotectonic unit and contains mainly Mesozoic carbonates, as well as Neogene, Pleistocene, and Holocene deposits (Gournelos et al., 2018; Fig. 1a). Geomorphologically, it can be divided into three main regions (Gournelos et al., 2018). (1) The northern, mountainous area contains the island's highest elevation (Pantokrator, with 906 m above sea level) and consists mainly of Mesozoic and Neogene carbonates and clastic sedimentary rocks (Triantaphyllou et al., 1995; Tserolas et al., 2016; Gournelos et al., 2018; Fig. 1a). (2) The central part contains Triassic breccias and folded Neogene deposits forming predominantly undulating landforms (Rögl et al., 1997; Tserolas et al., 2016; Gournelos et al., 2018). (3) The southern area is characterized by a low topography formed by Neogene and Quaternary successions, including recent sand dunes (Triantaphyllou et al., 1995; Rögl et al., 1997; Tserolas et al., 2016; Gournelos et al., 2018; Fig. 1a).
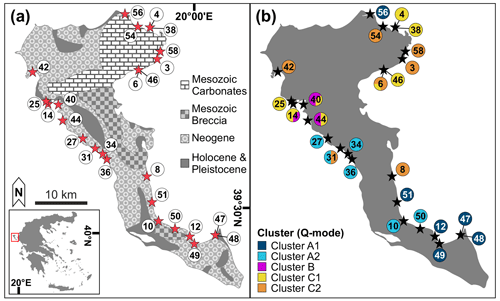
Figure 1Map of the sampling locations on Corfu Island. (a) Overview map of the 25 sampling stations; inlet: location of Corfu Island in Greece (modified from GinkgoMaps, http://www.ginkgomaps.com, last access: 27 May 2020). Geological information adapted from Tserolas et al. (2016). (b) Distribution of the sub-clusters from the Q-mode cluster analysis among the 25 sample stations (see Fig. 3). Different colors at the same location represent the portions of two clusters at that location.
The western coast faces the open Ionian Sea, and the seafloor drops quickly and steeply to more than 1000 m (Higgins, 2009). The eastern coast drops much more gently into the Corfu Channel. The channel width varies between 2 and 22 km, and the water depths barely exceed 100 m (Weinmann et al., 2019, and references therein). As such, the eastern and especially southeastern coast, which form the Gulf of Corfu, build a low-energy and protected environment, whereas the western coast is generally wind-exposed and prone to strong storm events – predominantly during the winter months (Fischer et al., 2016).
The shallow-water habitats around Corfu include sandy and silty areas with sparse vegetation of Zostera marina in the southeastern area. Towards the north, the sediments become coarser and the vegetation coverage is more extensive, including mixed meadows of Cymodocea nodosa and Zostera spp. In deeper water depths (> 3 m), extensive seagrass areas of Posidonia oceanica occur, especially along the central eastern coast between Corfu Town and Mesongi. The steeper, western coast is characterized by vegetated hard grounds (mostly with Cystoseira spp.) and sparsely vegetated sands in the south.
2.2 Sampling and sample processing
In May and October 2017, we collected a total of 51 sediment samples from 25 sampling stations around the island with 1–4 replicates per station (Fig. 1a; see field data in Weinmann et al., 2022). The sediment samples were dominated by different grain sizes and grouped into three categories based on the main grain size: fine sand/sand (22 samples), sand (13 samples), and sand/rubble (16 samples; see field data in Weinmann et al., 2022). The samples covered depths from 0.5–40 m and were grouped into three depth ranges: < 2 m (16 samples), 2–5 m (24 samples), and > 5 m (11 samples; see field data in Weinmann et al., 2022). They also included four different major habitats: sand (6 samples), hard ground (vegetated rubble or rocky ground, 12 samples), and seagrass patches (33 samples; see field data in Weinmann et al., 2022). Within the seagrass patches, samples were taken either on the periphery of the patch (20 samples) or within the center of the patch (13 samples).
Sampling of the material was performed by snorkeling or diving and filling ca. 50 mL of loose sediment from the topmost layer into 100 mL plastic containers with tight-fitting lids. After settling of the material, excess water was decanted and the containers were filled with a solution of 90 % ethanol and 2 g Rose Bengal per liter. Although less effective than other tracking methods, the application of Rose Bengal still sufficiently allows an estimation of the living population in shallow-water benthic foraminifera, especially in well-oxygenated environments (Murray and Bowser, 2000).
After a minimum of 2 weeks, the material was washed with tap water over a 63 µm sieve and oven-dried at 40 ∘C. A representative amount (∼ 300 specimens) from each sample was picked, and the foraminifera were identified and counted. For identification we used local catalogues (Cimerman and Langer, 1991; Hottinger et al., 1993; Milker and Schmiedl, 2012; Meriç et al., 2014; Dimiza et al., 2016a; Mouanga, 2018) and the World Foraminifera Database (http://www.marinespecies.org/foraminifera, last access: 21 June 2022; Hayward et al., 2022) to ensure the use of the most recent nomenclature. We picked total assemblages (stained and dead specimens) to provide time-averaged information on the foraminiferal assemblages. The use of total assemblages preserved in the sediment samples avoided bias from the short life cycles and spatial variability of some shallow-water foraminifera and thus allowed for a better representation and evaluation of local communities in response to environmental conditions throughout the year (Langer and Lipps, 2003; Debenay et al., 2005; Langer and Mouanga, 2016; Dimiza et al., 2018; Sariaslan and Langer, 2021).
2.3 Data analyses
We calculated several community parameters to evaluate the assemblage structure of the benthic foraminifera. Parameters included species richness (S), Fisher α, Shannon (H) index, and Berger–Parker dominance index (max pi). Fisher α is defined as
where S is the number of taxa (species richness) and N is the number of individuals (Fisher et al., 1943). The Shannon (H) index, also known as the information function index (Shannon, 1948), is defined as
with pi being the proportion of the ith taxon. The Berger–Parker dominance index (max pi) is defined as the proportion of the most abundant species (Berger and Parker, 1970; see also Hayek and Buzas, 2013).
Ternary plots were calculated on the basis of relative abundances of the three wall types in polythalamous foraminifera (hyaline, porcelaneous, and agglutinated) for the entire fauna. For further analyses, we selected species with high abundances (> 3 % in at least one sample) and/or wide distributions among the samples (present in at least one-third of the samples). A preliminary R-mode cluster analysis of species meeting either criterion or both of these criteria revealed a distinct assemblage cluster with 76 species, and these – together with Amphistegina lobifera – were used for all further analyses. The raw count data for the 77 selected species were log-transformed, and a Q-mode cluster analysis was performed based on the Euclidean distance and Ward's method. The same set of 77 species was used to calculate relative abundances of six life modes of benthic foraminifera (Table 1; see count data in Weinmann et al., 2022): epifaunal, infaunal, permanently attached epiphytic (type A), temporarily attached epiphytic (type B), temporarily motile epiphytic (type C), and permanently motile epiphytic (type D). The six groups were determined on the same basis as in Weinmann et al. (2019, and references therein).
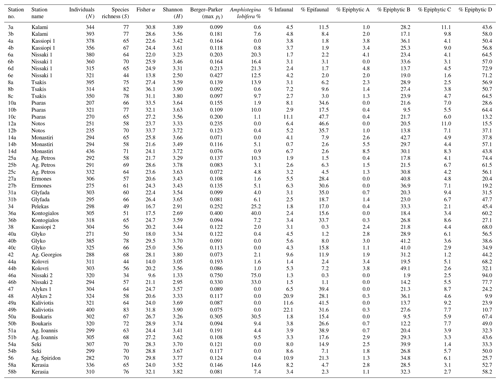
Table 1Community parameters of benthic foraminifera at all sampled stations. Diversity indices and Amphistegina lobifera abundances are for the entire benthic assemblages. Relative abundances of life modes were calculated for the 77 most abundant species that were used in other analyses.
The Q-mode cluster analysis based on the characterizing species was used since we expected the assemblages to fall into different groups (see Murray, 2006) based on the environmental differences observed around the island. Ternary plots of wall types have long been used in ecological studies (see Murray, 1973) as they provide insight into environmental differences. For the different life modes of foraminifera we applied a principal component analysis (PCA). The ternary plots and the PCA were conducted to validate the groups provided by the cluster analysis and to provide several lines of evidence for the variable environments represented by the foraminiferal assemblages of Corfu. All analyses were further supported by analysis of similarity (ANOSIM) or analysis of variance (ANOVA) to validate the significance of the observed groupings.
Calculation of community parameters (e.g., diversity indices), cluster analysis, ternary plots, principal component analysis (PCA), and correlation analyses was performed with PAST (Paleontological Statistics v. 4.02; Hammer et al., 2001). Representative specimens of the most abundant species were imaged with a scanning electron microscope (SEM; NeoScope JCM-5000). The resulting figures were put together in a plate with GIMP (2.10.14, http://gimp.org, last access: 13 May 2020). All other graphics including maps were prepared with CorelDRAW (X7, http://coreldraw.com, last access: 13 May 2020).
3.1 Foraminiferal fauna and diversity
In total, 17 164 specimens were counted, including 16 139 benthic and 1025 planktonic specimens. The number of benthic individuals (N) per sample ranged from 207 to 436, with 316 on average (Table 1). The planktonic specimens accounted for 0 %–51 % of the foraminiferal assemblages (5 % on average; see count data in Weinmann et al., 2022) and showed a heterogeneous distribution. They were absent from many samples and showed their highest values in shallow-water samples from the southeastern part of the island. Between 0.3 % and 43 % of the encountered specimens per sample were stained (6.6 % on average; see count data in Weinmann et al., 2022). We identified 200 species of benthic foraminifera, belonging to 96 genera (see inventory of foraminifera in Weinmann et al., 2022). Of these, 93 species were porcelaneous, 92 species were hyaline perforate, and 15 species were agglutinated. The most commonly encountered taxa are illustrated in Fig. 2.
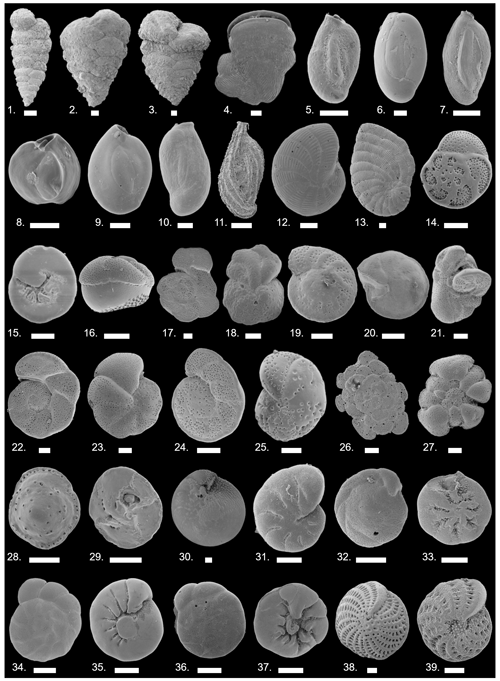
Figure 2Plate depicting the most abundant foraminiferal species around Corfu Island. All scale bars are 100 µm. (1) Textularia agglutinans; (2) T. bocki; (3) T. lateralis; (4) Vertebralina striata; (5) Quinqueloculina parvula; (6) Q. seminulum; (7) Q. schlumbergeri; (8) Q. vulgaris; (9) Pseudotriloculina rotunda; (10) Pseudotriloculina. sp. 1; (11) Sigmoilina costata; (12) Peneroplis pertusus; (13) P. planatus; (14) Rosalina bradyi, spiral view; (15) R. bradyi, umbilical view; (16) Tretomphalus bulloides; (17) Lobatula lobatula, spiral view; (18) L. lobatula, umbilical view; (19) Cibicidoides cf. subhaidingerii, spiral view; (20) C. cf. subhaidingerii, umbilical view; (21) C. variabilis; (22) Cibicides advenum, spiral view; (23) C. advenum, umbilical view; (24) C. refulgens, spiral view; (25) C. refulgens, umbilical view; (26) Planorbulina mediterranensis, spiral view; (27) P. mediterranensis, umbilical view; (28) Asterigerinata mamilla, spiral view; (29) A. mamilla, umbilical view; (30) Amphistegina lobifera; (31) Haynesina depressula; (32) Buccella sp. 1, spiral view; (33) B. sp. 1, umbilical view; (34) Ammonia parkinsoniana, spiral view; (35) A. parkinsoniana, umbilical view; (36) A. tepida, spiral view; (37) A. tepida umbilical view; (38) Elphidium crispum; (39) E. macellum.
Species richness (S) ranged between 34 and 83 (64.6 on average, Table 1), the Fisher α index varied between 9.6 and 36.1 (25 on average, Table 1), the Shannon (H) index varied between 1.33 and 3.90 (3.46 on average, Table 1), and the Berger–Parker dominance max pi varied between 0.07 and 0.75 (0.16 on average, Table 1).
3.2 Cluster analysis
The Q-mode cluster analysis resulted in three main environmental clusters (A–C, Fig. 3) and five sub-clusters (A1, A2, B, C1, C2). Cluster A combined 20 samples that mostly originated from the southern part of the island, with the exception of sample 56 (Ag. Spiridon, Fig. 1b). It included all but two samples from the shallowest depth range (< 2 m) and no samples deeper than 5 m (Fig. 3). It was also strongly dominated by the finest grain size fraction. Cluster A1 contained all of the six sandy habitats, while cluster A2 contained the highest number of hard-ground samples (Fig. 3). Cluster B combined only four samples from the central western part of the island (Fig. 1b). All samples came from depths deeper than 5 m, contained mostly coarse sediments, and included two hard-ground and two seagrass samples (Fig. 3). Cluster C combined all remaining samples from the northwest, the north, and northeast of the island as well as sample station 8 (Tsakis, Fig. 1b) at the southeastern coast of Corfu. The majority of samples originated from intermediate depths (2–5 m) and seagrass habitats (both peripheral and central, Fig. 3). Cluster C1 was represented by a higher number of hard-ground samples, and the sediments contained mostly medium to coarse sand and rubble (Fig. 3).
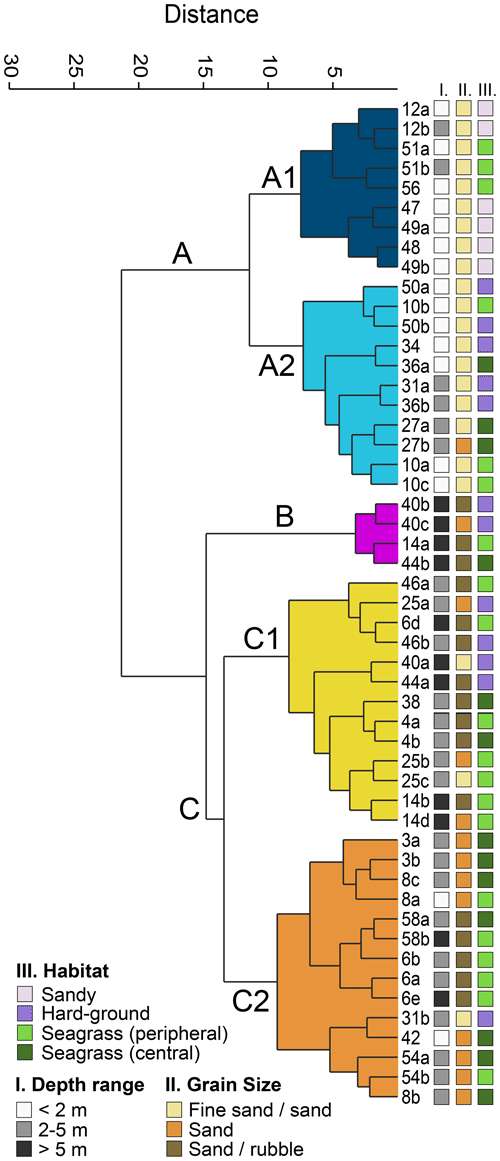
Figure 3Q-mode cluster analysis based on the 77 most abundant foraminifera from Corfu Island. The three depth range groups represent the sampled water depth. The three grain size groups represent the dominant size fractions in the sediments. The four habitat groups represent the sampled environments.
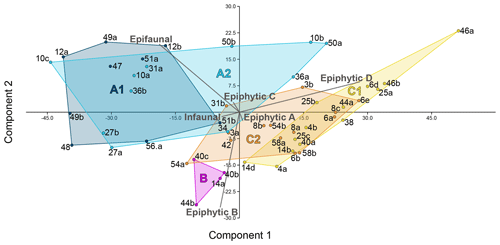
3.3 Distribution of wall types and life modes
All three foraminiferal wall types expressed as relative abundances were plotted in a ternary diagram and overlain with the five environmental sub-clusters (A1–C2, Fig. 4). The diagram shows an overall dominance of hyaline-perforate taxa (> 40 % for 48 samples). It also reveals that the samples from cluster B and C contained significantly higher numbers of porcelaneous taxa than the samples from cluster A. The relative number of agglutinated taxa was higher in sub-clusters A2 and C1, compared to the others. Differences between the sub-clusters were statistically significant (Table 2).
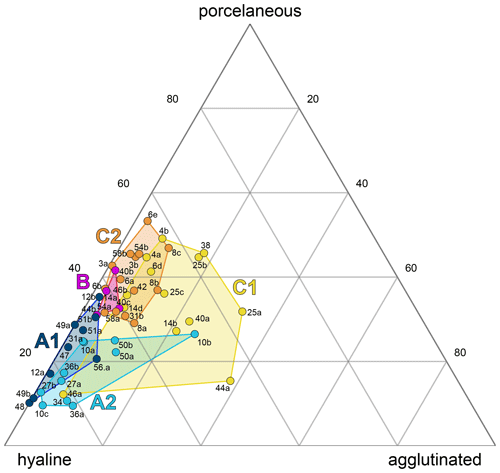
Figure 4Ternary plot of the wall types of polythalamous foraminifera from Corfu Island. Superimposed are the cluster groups from Fig. 3.
Table 2ANOSIM for the groups defined in the Q-mode cluster analysis compared to wall types and life modes.
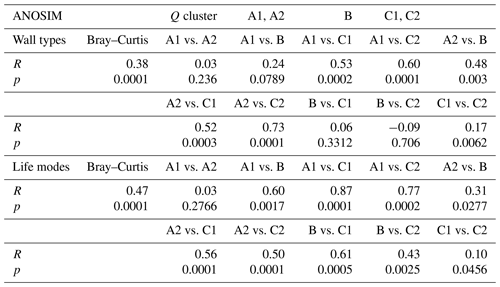
For the analysis of the distribution of the six life modes among the foraminiferal assemblages, a principal component analysis (PCA) was performed (Fig. 5). With eigenvalues of 514.067 and 143.24 respectively, the two main components together explained 97 % of the variance. On the first axis, the diagram separates the assemblages based on the different abundances of epifaunal and permanently motile epiphytic (type D) taxa. This reveals the most significant differences between cluster A and cluster C (Fig. 5). On the second axis, the main separator is the group of temporarily attached epiphytic (type B) taxa, which characterize cluster B (Fig. 5). The differences between the overlain groups based on the five environmental sub-clusters were significant (Table 2).
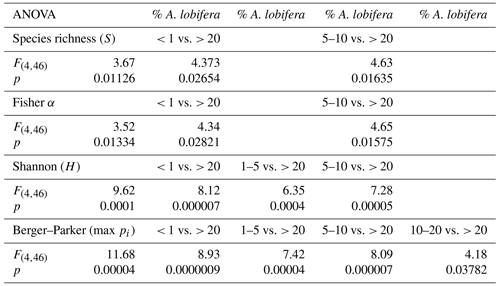
3.4 Effects of the relative abundance of Amphistegina lobifera
Amphistegina lobifera was present in 40 out of 51 samples (78 %, Table 1) with relative abundances between 0.1 and 75 %. In every fourth sample, the species already accounted for more than 10 % (Table 1). Based on these numbers, we grouped the relative abundances of A. lobifera among the foraminiferal assemblages of the samples into five groups: < 1 %, 1 %–5 %, 5 %–10 %, 10 %–20 %, and > 20 %. Higher abundances of A. lobifera had an influence on the faunal diversity. ANOVA revealed that Shannon diversity (H) was significantly lowered with increasing relative abundance of A. lobifera, especially if the latter exceeded 20 % (Tables 1 and 3). The same trend was observed for the Berger–Parker dominance (max pi) within the samples (Tables 1 and 3). Compared to that, the species richness (S) and Fisher α were less affected by the presence of A. lobifera (Tables 1 and 3). The only statistically significant effects were detected between the lowest (< 1 %) and medium abundances (5 %–10 %) compared to the highest abundances (> 20 %) of A. lobifera (Tables 1 and 3).
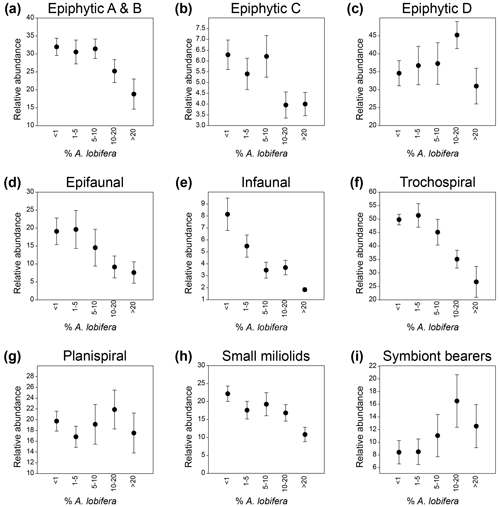
We plotted the relative abundances of specific groups among the 76 species of other foraminifera that were used in the cluster analysis and PCA against the five groups of the A. lobifera abundance to evaluate the effect of the latter on other taxa (Fig. 6). We also plotted the mean abundances of the same groups against the mean abundance of A. lobifera within each of the five groups and calculated the correlation values (Pearson correlation). Results showed that attached epiphytic taxa (types A and B combined) showed a significant negative correlation with increasing abundances in A. lobifera (R = −0.967, p = 0.0071; Fig. 6a). The same trend could be observed for small miliolid taxa (R = −0.943, p = 0.0163; Fig. 6h) and other trochospiral taxa (without the trochospiral A. lobifera, R = −0.944, p = 0.0157; Fig. 6f). Negative correlations were also observed for epifaunal and infaunal taxa, although with lower significance (R = −0.857, p = 0.0637 for epifaunal and R = −0.811, p = 0.0961 for infaunal taxa; Fig. 6d and e). On the other hand, permanently motile epiphytic taxa (type D, without the permanently motile A. lobifera) and symbiont-bearing taxa (without the symbiont-bearing A. lobifera) revealed a positive correlation with increasing abundance of A. lobifera until the latter exceeded 20 % of the total assemblage (R = 0.953, p = 0.0122 for epiphytic D and R = 0.977, p = 0.0042 for symbiont-bearing taxa; Fig. 6c and i). In samples where A. lobifera exceeded 20 %, the median relative abundances of both groups were significantly lower (Fig. 6c and i). Taxa belonging to the temporarily motile epiphytic group C (mostly Elphidium spp.) did not show a linear correlation with Amphistegina abundances (Fig. 6b). Nevertheless, the relative abundances of these taxa were significantly lower in samples where A. lobifera exceeded 10 %. Only the planispiral taxa showed no correlation to the abundances of A. lobifera (Fig. 6g).
4.1 Spatial distribution and diversity of benthic foraminifera from Corfu Island
Despite the limited depth distribution of samples, we found a high diversity in benthic foraminifera with 200 species in total assemblages. The number was comparable to or higher than other studies on total foraminiferal assemblages from adjacent regions and/or comparable water depths: Mouanga (2018) found 277 species along the coast of Albania in 0.5–30 m, Debenay et al. (2005) reported 147 species from the Gulf of Kalloni (Lesvos) in 5–25 m, Aiello et al. (2006) found 111 species in Porto Cesareo lagoon (southern Italy) between 0.12–4.78 m, Dimiza et al. (2016a) reported 78 species from very shallow sites in three gulfs of the western Aegean Sea, and Dimiza et al. (2018) found 78 species in the Saronikos Gulf (also known as the Saronic Gulf) in 8–29 m, although the latter site is influenced by different levels of pollution. This corroborates our initial hypothesis that Corfu harbors a relatively high diversity in shallow-water foraminifera. Other diversity indices further support this. The Fisher α values that we found in Corfu (9.6–36.1) were comparable to values reported from Albania (7.2–28.7; Mouanga, 2018) but higher compared to the Saronikos Gulf and other gulfs of the western Aegean Sea (1.3–17.3; Dimiza et al., 2016a, 2018). The Shannon (H) index values that we reported (1.33–3.9) are well within the ranges of other areas from the Mediterranean, such as Albania (1.2–3.8, Langer and Mouanga, 2016), Porto Cesareo lagoon (1.8–3.2; Aiello et al., 2006), the western Aegean gulfs (1.0–3.3; Dimiza et al., 2016a, 2018), or the shallower areas of the Adriatic shelf (1.6–2.6; Capotondi et al., 2015).
The relatively high diversity in shallow-water areas of Corfu Island and Albania could, on the one hand, be attributed to their position in the transition zone between the eastern and western Mediterranean Sea (Mouanga, 2018). With ongoing ocean warming, continuous species introductions from both basins are expected, which would increase local species richness. We found several species that have been defined as non-indigenous in the Mediterranean (Stulpinaite et al., 2020): Amphistegina lobifera, Euthymonacha polita, Miliolinella cf. fichteliana, Quinqueloculina cf. multimarginata, Sigmoihauerina bradyi, and Spiroloculina antillarum (see count data and inventory of foraminifera in Weinmann et al., 2022). These are accompanied by taxa that are native to the Mediterranean but have been confined to the western or eastern basins so far. On the other hand, the high variability of shallow-water habitats in this area (coarse and medium sand, hard-ground/rocky shore, and extended vegetation cover) also increases local diversity of benthic foraminifera.
Analyses of foraminiferal distribution and environmental parameters revealed foraminiferal assemblages that were primarily influenced by water depth with significant differences in samples from < 2 or > 5 m. Secondarily, the habitat type played a role, especially between sandy and vegetated areas. We were able to distinguish three main environmental clusters along the coasts of Corfu Island.
-
Cluster A covers the southern island with mostly shallow water depths. In particular, sub-cluster A1 contains mostly fine sand to sandy sediments from the eastern side, while sub-cluster A2 contains hard-ground areas on the western side. The assemblages are dominated by hyaline taxa and mostly epifaunal, infaunal, and temporarily attached epiphytic species (type C). Especially the assemblages from sub-cluster A1 are probably favored by the low-energy, protected environment of the Gulf of Corfu on the eastern coast of the island, which faces the overall shallow and low-energetic Corfu Channel towards the mainland. On the western side, the samples were taken in small bays which face the open Ionian Sea but are still protected from stronger wind and wave regimes. Still, the specimens from these sites often exhibit a higher number of abraded shells compared to the eastern side of the island. The entire cluster A assemblage is comparable to those from northern Albania (Mouanga, 2018); silty–sandy sediments from Sicily (Guastella et al., 2019); sheltered areas from Porto Cesareo lagoon (Aiello et al., 2006); the nearshore fauna of the Adriatic shelf (Capotondi et al., 2015); or sandy areas from the Vistonikos, Thermaikos, and South Evoikos gulfs (western Aegean; Dimiza et al., 2016a). Epifaunal, hyaline taxa were also the dominant species in the Gulf of Kalloni (Lesvos; Debenay et al., 2005).
-
Cluster B is restricted to a few samples from deeper water depths on the western coast, where they were taken from sediment pockets in steeply declining walls. The cluster is dominated by temporarily attached epiphytes (type B) and highly diverse small miliolids. The greater depths of these habitats allow for protection from the higher-energy environments and regular storm events of the western coast. Their association with coarser sediments is typical for epiphytic taxa (Barras et al., 2014). The assemblages belonging to cluster B are comparable to those from southern Albania (Langer and Mouanga, 2016; Mouanga, 2018); algal samples from Sicily (Guastella et al., 2019); Caulerpa racemosa habitats from the Balearic Islands (Mateu-Vicens et al., 2010, 2014); and the unpolluted, vegetated substrates of the Saronikos Gulf (Dimiza et al., 2018). Their overall composition is quite comparable to the Pleistocene assemblages from the south of Corfu (Maragoudakis, 1967; Rögl et al., 1997).
-
Cluster C is mostly found in the northern island with medium to greater depths along rocky coasts and extended seagrass habitats. Especially in the northeastern part it includes almost all sample stations along the Mesozoic carbonate formations (Fig. 1). The cluster is dominated by permanently motile epiphytic taxa (type D), especially diverse small miliolids, but also symbiont-bearing foraminifera (mainly Peneroplis spp. and partially high abundances in A. lobifera) and textulariids. It includes the highest abundances of porcelaneous taxa. The northern part of the island is characterized by a quite extensive drainage system of small streams which drain the calcareous Mesozoic and Neogene formations that characterize the area (Gournelos et al., 2018; Fig. 1a). High amounts of eroded and drained carbonates together with relatively quiet environments could lead to the proliferation of porcelaneous foraminifera in these coastal areas of northern Corfu. Comparable assemblages to cluster C include the southern rocky shores of Albania (Langer and Mouanga, 2016; Mouanga, 2018), shallow marine communities of the Thermaikos and South Evoikos gulfs (western Aegean; Dimiza et al., 2016a), and other vegetated assemblages from the Aegean and central Mediterranean (Triantaphyllou et al., 2005; Koukousioura et al., 2010, 2011; Guastella et al., 2019).
Our analysis shows that (1) the unique geography of Corfu Island with its three major geomorphological zones and (2) the habitat distribution with its highly variable shallow-water environments provide excellent conditions for the development of diverse shallow-water foraminiferal faunas.
4.2 Effects of Amphistegina lobifera on the foraminiferal assemblages at Corfu Island
Our study showed that Amphistegina lobifera was a common or dominant taxon on vegetated hard grounds (e.g., in association with Cystoseira) and in seagrass habitats (Cymodocea nodosa and Posidonia oceanica). Its abundance was lowest in the sandy, less-vegetated, shallow-water areas of the southeastern Gulf of Corfu and in the deep settings represented by cluster B. With its highest abundances in the northeastern part of the island, it appears that the geomorphology with its three distinct zones has a larger influence on Amphistegina proliferation than the differences between the steeper western and shallower eastern coasts.
Percent abundances of Amphistegina lobifera generally remained below 20 %, and only two sites revealed extreme abundances of 40 % and 75 % respectively (Table 1). At low levels, we found that there was no ubiquitously negative effect of the presence of Amphistegina lobifera on the local diversity of shallow-water foraminiferal assemblages from Corfu Island. While the Shannon (H) index and dominance were initially affected by increasing abundances of A. lobifera, the negative impact on species richness (S) and Fisher α values was only significant when A. lobifera exceeded 20 % of the total assemblage. At low levels, A. lobifera appears to have no or only minor effects on local foraminiferal assemblages (Koukousioura et al., 2010). At extremely high levels (75 %, site 46a), however, species richness and Fisher α values are reduced to their lowest values and show a clear impact of the invasive species. This agrees with previous studies that showed significant effects of high numbers of A. lobifera on native assemblages and the local foraminiferal diversity: Mouanga and Langer (2014) reported a 30 % diversity reduction when Amphistegina exceeded 25 %. In addition, locally extreme abundances of A. lobifera (∼ 87 %) reduced species richness and diversity of autochthonous assemblages in Linosa (southern Italy; Caruso and Cosentino, 2014).
We found that higher abundances of A. lobifera had a larger effect on the relative abundances of certain foraminiferal groups. Attached epiphytic taxa (types A and B) and small miliolids are most affected. This was also described by Langer and Mouanga (2016), where the number of epiphytes decreased when Amphistegina abundances exceeded 9 % and small miliolids were least abundant with Amphistegina surpassing 16 %. Both groups inhabit the same habitats as A. lobifera and might be outcompeted by its increasing numbers, which could lead to a displacement of taxa from similar habitats and competitive exclusion (Langer and Mouanga, 2016). Permanently attached epiphytes have longer life spans (Langer, 1988, 1993; Mateu-Vicens et al., 2010) and might be at a disadvantage compared to A. lobifera, which can reproduce quickly compared to other larger benthic foraminifera. On the other hand, other symbiont-bearing taxa show a positive correlation with A. lobifera until the latter exceeds 20 %. Their shared preferences for warm temperatures and oligotrophic conditions let them thrive comparably before A. lobifera becomes too dominant. This could suggest a general faunal shift in the future with ongoing ocean warming. Interestingly, Guastella et al. (2021) showed that in Malta, A. lobifera has increased significantly in abundance since the early 1990s when ocean temperatures became significantly warmer.
A previous study of foraminiferal assemblages from the southern coast of Corfu in the Pleistocene revealed a prevalence of attached epiphytes (mainly type B) and small miliolids (Rögl et al., 1997). In our analysis, these were the taxa that are currently most affected by the increasing presence of Amphistegina lobifera. On the other hand, Rögl et al. (1997) reported that peneroplids and other warm-water taxa were very rare during the Pleistocene around Corfu. In addition, peneroplids have not been documented from Pliocene deposits in the southwestern part of Corfu (Triantaphyllou et al., 1995). Peneroplids together with Amphistegina and the tropical textulariids could be better adapted to the ongoing increasing temperatures, which has been described by Triantaphyllou et al. (2012) as a previously vacant niche for symbiont-bearing foraminifera that is augmented by rising temperatures. We observed a good co-existence of these taxa in the warmer seagrass meadows until A. lobifera became too dominant (> 20 %). Motile epiphytes might also be at an advantage with being able to move away from light stress (e.g., more sun hours) in combination with warmer temperatures. Overall, symbiont-bearing taxa could become important contributors of the tropicalization of the central Mediterranean, which is specified as the appearance and spread of tropical (thermophilic) biota (Bianchi et al., 2012). With some observed adaptive mechanisms to extreme temperatures (e.g., Weinmann and Langer, 2017), A. lobifera might be able to further flourish under future scenarios.
Observations from the situation in the eastern Mediterranean can give insights into the possible future of Corfu Island. Some areas of the eastern Mediterranean are already evolving into novel ecosystems, which might be irreversible (e.g., Albano et al., 2021). This might already be true for particular sites that show very high abundances of amphisteginids (e.g., Meriç et al., 2008; Mouanga and Langer, 2014). However, for the moment, the good ecological status of the Corfu sites shows that despite the species invasion of Amphistegina lobifera, diversity can still be high, although certain groups are clearly affected. There are still samples from greater depths on the western coast, where the attached epiphytes dominate (cluster B), which are comparable to the Pliocene and Pleistocene biotas. However, this might be a local effect, since temporal upwelling after storm events (personal observation) could provide overall cooler temperatures, which might serve as potential local refuge areas for these taxa. This could mean that the local diversity of Corfu Island might be preserved in some areas, even under ongoing warming.
Further success might also depend on the status of Posidonia oceanica meadows in Corfu, which have been described as an ideal environment for the proliferation and expansion of Amphistegina (El Kateb et al., 2018). It has been shown that under good conditions A. lobifera can increase in abundance within a few years (from 0 in 2014 to 12 % in 2016 and 50 % in 2017; Caruso and Cosentino, 2014; Guastella et al., 2019), highlighting its successful adaptation to local conditions (Triantaphyllou et al., 2009; Koukousioura et al., 2010). Its invasiveness increases over time, and Amphistegina becomes the most abundant taxon after 1–2 decades to attain values between 10 % and 50 % (Mouanga and Langer, 2014). The numbers around Corfu Island would suggest that the proliferation of A. lobifera in this region probably started around the same time as in Malta, although the exact arrival date is unknown (Guastella et al., 2021).
Attention should be drawn to the role of Amphistegina lobifera as an ecosystem engineer. The increasing accumulation of its nearly spherical, sand-sized, and robust tests leads to alterations of local sediments (Langer et al., 2012, and references therein; Triantaphyllou et al., 2012) and also provides new attachment surfaces for other organisms (Langer and Mouanga, 2016). New observations on some seagrass habitats in Malta showed that A. lobifera now constitutes a significant part of the bottom sediment by forming layers that exceed several centimeters in thickness and profoundly changes the local seabed character (Vohník, 2021). The same study also found that empty shells of A. lobifera are regularly bioeroded by assemblages of microborers, mycobionts, and epiphytes, whose composition differs from those of adjacent bioeroded substrates, such as roots of Posidonia oceanica (Vohník, 2021). These observations support the hypothesis that the proliferation of amphisteginid foraminifera may ultimately lead to changes in ecosystem functioning (e.g., Langer et al., 2012; Langer and Mouanga, 2016; Mouanga, 2018).
In some eastern Mediterranean sites, the foraminiferal assemblages are described as resembling monocultures of Amphistegina (Langer and Mouanga, 2016), e.g., comprising more than 70 % of the foraminiferal shells in Türkiye (previously Turkey; Meriç et al., 2008; Yokeş et al., 2014; Mouanga and Langer, 2014) and 60 %–100 % in Cyprus (Abu Tair and Langer, 2010; Mouanga and Langer, 2014). The resulting sediments are already beginning to resemble the Amphistegina limestones of certain Pliocene locations in Italy (e.g., Di Bella et al., 2005; Coletti et al., 2021), which have been described as offering potential insight into the future of the Mediterranean (Coletti et al., 2021). Another example for such monocultures is the nearly monospecific lenticular Nummulites facies associated with the Paleocene–Eocene Thermal Maximum in the Tethys Ocean (Hallock et al., 2011). It can not be determined today if current Mediterranean sediments will be described as Amphistegina limestones again in the future. But the current observations allow a better understanding of the time frames and mechanisms behind such mass occurrences of the past.
The present study, with its current faunal inventory and its quantification of the effects of Amphistegina lobifera on the local assemblage, can serve as a baseline from the early to median stage of the A. lobifera invasion in an ecosystem (∼ 20 years) and its establishment in local assemblages. As such, it will certainly benefit future studies that further monitor and analyze the ongoing effects of A. lobifera under the prospected tropicalization of the central Mediterranean region.
The datasets that were generated and analyzed for this study are available in the NHMW Data Repository: https://doi.org/10.57756/9uhty3 (Weinmann et al., 2022).
AEW and MRL conceived the study and designed the methodology; AEW performed fieldwork (with support from MVT); AEW, OK, MVT, and MRL analyzed the data and wrote the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We wish to thank Michael Kunert for field assistance, Michael Freise for providing samples, and Nadine Blume for laboratory assistance and picking of foraminifera. We further acknowledge Iris Feichtinger and Oleg Mandic for assistance with SEM imaging. We are thankful to Abduljamiu Amao and Nicoletta Mancin as well as the editor Sergio Navarrete for their thoughtful reviews and constructive comments and suggestions.
This research has been supported by the Deutsche Forschungsgemeinschaft (grant no. 323009980).
This paper was edited by Sergio Navarrete and reviewed by Nicoletta Mancin and Abduljamiu Amao.
Abu Tair, N. K. and Langer, M. R.: Foraminiferal invasions: the effect of Lessepsian migration on the diversity and composition of benthic foraminiferal assemblage around Cyprus (Mediterranean Sea): Forams 2010 – International Symposium on Foraminifera, Bonn, Germany, 5–10 September 2010, Abstracts, Rheinische Friedrich-Wilhelms-Universität Bonn, p. 42, 2010.
Aiello, G., Barra, D., Coppa, M. G., Valente, A., and Zeni, F.: Recent infralittoral Foraminiferida and Ostracoda from Porto Cesareo Lagoon (Ionian Sea, Mediterranean), B. Soc. Paleontol. Ital., 45, 1–14, https://www.paleoitalia.it/wp-content/uploads/2022/04/001_014_AIELLO.pdf (last access: 31 August 2021), 2006.
Albano, P. G., Steger, J., Bošnjak, M., Dunne, B., Guifarro, Z., Turapova, E., Hua, Q., Kaufman, D. S., Rilov, G., and Zuschin, M.: Native biodiversity collapse in the eastern Mediterranean, P. Roy. Soc. B. Biol. Sci., 288, 20202469, https://doi.org/10.1098/rspb.2020.2469, 2021.
Barras, C., Jorissen, F. J., Labrune, C., Andral, B., and Boissery, P.: Live benthic foraminiferal faunas from the French Mediterranean Coast: Towards a new biotic index of environmental quality, Ecol. Indic., 36, 719–743, https://doi.org/10.1016/j.ecolind.2013.09.028, 2014.
Berger, W. H. and Parker, F. L.: Diversity of planktonic foraminifera in deep-sea sediments, Science, 168, 1345–1347, https://doi.org/10.1126/science.168.3937.1345, 1970.
Bianchi, C. N.: Biodiversity issues for the forthcoming tropical Mediterranean Sea, Hydrobiologia, 580, 7–21, https://doi.org/10.1007/s10750-006-0469-5, 2007.
Bianchi, C. N., Morri, C., Chiantore, M., Montefalcone, M., Parravicini, V., and Rovere, A.: Mediterranean Sea biodiversity between the legacy from the past and a future of change, in: Life in the Mediterranean Sea: A look at habitat changes, edited by: Stambler, N., Nova Science Publishers, New York, 1–56, ISBN 978-1-61209-644-5, 2012.
Capotondi, L., Bergami, C., Orsini, G., Ravaioli, M., Colantoni, P., and Galeotti, S.: Benthic foraminifera for environmental monitoring: A case study in the central Adriatic continental shelf, Environ. Sci. Pollut. R., 22, 6034–6049, https://doi.org/10.1007/s11356-014-3778-7, 2015.
Caruso, A. and Cosentino, C.: The first colonization of the Genus Amphistegina and other exotic benthic foraminifera of the Pelagian Islands and south-eastern Sicily (central Mediterranean Sea), Mar. Micropaleontol., 111, 38–52, https://doi.org/10.1016/j.marmicro.2014.05.002, 2014.
Cimerman, F. and Langer, M. R.: Mediterranean foraminifera, Razred za naravoslovne vede, classis IV, historia naturalis, opera 30, Slovenska Akademia, Ljubljana, 118 pp., ISBN 8671310531, 1991.
Coletti, G., Bosio, G., and Collareta, A.: Lower Pliocene barnacle facies of western Liguria (NW Italy): A peek into a warm past and a glimpse of our incoming future, Riv. It. Paleontol. Strat., 127, 103–131, https://doi.org/10.13130/2039-4942/15202, 2021.
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Ben Rais Lasram, F., Aguzzi, J., Ballesteros, E., Bianchi, C. N., Corbera, J., Dailianis, T., Danovaro, R., Estrada, M., Froglia, C., Galil, B. S., Gasol, J. M., Gertwagen, R., Gil, J., Guilhaumon, F., Kesner-Reyes, K., Kitsos,, M.S., Koukouras, A., Lampadariou, N., Laxamana, E., López-Fé de la Cuadra, C. M., Lotze, H. K., Martin, D., Mouillot, D., Oro, D., Raicevich, S., Rius-Barile, J., Saiz-Salinas, J. I., San Vicente, C., Somot, S., Templado, J., Turon, X., Vafidis, D., Villanueva, R., and Voultsiadou, E.: The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats, PLoS One, 5, e11842, https://doi.org/10.1371/journal.pone.0011842, 2010.
Debenay, J.-P., Millet, B., and Angelidis, M. O.: Relationships between foraminiferal assemblages and hydrodynamics in the Gulf of Kalloni, Greece, J. Foramin. Res., 35, 327–343, https://doi.org/10.2113/35.4.327, 2005.
Di Bella, L., Carboni, M. G., and Pignatti, J.: Paleoclimatic significance of Pliocene Amphistegina levels from the Tyrrhenian margin of Central Italy, B. Soc. Paleontol. Ital., 44, 219–229, 2005.
Di Lorenzo, M., Sinerchia, M., and Colloca, F.: The North sector of the Strait of Sicily: A priority area for conservation in the Mediterranean Sea, Hydrobiologia, 821, 235–253, https://doi.org/10.1007/s10750-017-3389-7, 2018.
Dimiza, M. D., Koukousioura, O., Triantaphyllou, M. V., and Dermitzakis, M. D.: Live and dead benthic foraminiferal assemblages from coastal environments of the Aegean Sea (Greece): Distribution and diversity, Rev. Micropaleontol., 59, 19–32, https://doi.org/10.1016/j.revmic.2015.10.002, 2016a.
Dimiza, M. D., Triantaphyllou, M. V., Koukousioura, O., Hallock, P., Simboura, N., Karageorgis, A. P., and Papathanasiou, E.: The Foram Stress Index: A new tool for environmental assessment of soft-bottom environments using benthic foraminifera. A case study from the Saronikos Gulf, Greece, Eastern Mediterranean Sea, Ecol. Indic., 60, 611–621, https://doi.org/10.1016/j.ecolind.2015.07.030, 2016b.
Dimiza, M. D., Ravani, A., Kapsimalis, V., Panagiotopoulos, I. P., Skampa, E., and Triantaphyllou. M. V.: Benthic foraminiferal assemblages in the severely polluted coastal environment of Drapetsona-Keratsini, Saronikos Gulf (Greece), Rev. Micropaleontol., 62, 33–44, https://doi.org/10.1016/j.revmic.2018.09.001, 2018.
El Kateb, A., Stalder, C., Stainbank, S., Fentimen, R., and Spezzaferri, S.: The genus Amphistegina (benthic foraminifera): distribution along the southern Tunisian coast, BioInvasions Rec., 7, 391–398, https://doi.org/10.3391/bir.2018.7.4.06, 2018.
Fischer, P., Finkler, C., Röbke, B. R., Baika, K., Hadler, H., Willershäuser, T., Rigakou, D., Metallinou, G., and Vött, A.: Impact of Holocene tsunamis detected in lagoonal environments on Corfu (Ionian Islands, Greece). Geomorphological, sedimentary and microfaunal evidence, Quatern. Int., 401, 4–16, https://doi.org/10.1016/j.quaint.2015.07.019, 2016.
Fisher, R. A., Corbet, A. S., and Williams, C. B.: The relation between the number of species and the number of individuals in a random sample of an animal population, J. Animal Ecol., 12, 42–58, https://doi.org/10.2307/1411, 1943.
Gournelos, T., Evelpidou, N., Karkani, A., and Kardara, E.: Recognition of erosion risk areas using Neural Network Technology. An application to the Island of Corfu, Rev. Geomorfol., 20, 56–65, https://doi.org/10.21094/rg.2018.020, 2018.
Guastella, R., Marchini, A., Caruso, A., Cosentino, C., Evans, J., Weinmann, A. E., Langer, M. R., and Mancin, N.: “Hidden invaders” conquer the Sicily Channel and knock on the door of the Western Mediterranean Sea, Estuar. Coast. Shelf S., 225, 106234, https://doi.org/10.1016/j.ecss.2019.05.016, 2019.
Guastella, R., Marchini, A., Caruso, A., Evans, J., Cobianchi, M., Cosentino, C., Langone, L., Lecci, R., and Mancin, N.: Reconstructing Bioinvasion Dynamics Through Micropaleontologic Analysis Highlights the Role of Temperature Change as a Driver of Alien Foraminifera Invasion, Front. Mar. Sci., 8, 675807, https://doi.org/10.3389/fmars.2021.675807, 2021.
Hallock, P., Triantaphyllou, M. V., Dimiza, M. D., and Koukousioura, O.: An invasive foraminifer in coastal ecosystems of the Eastern Mediterranean: Implications for understanding larger foraminiferal-dominated biofacies in the Cenozoic, 2011 GSA Annual Meeting, Minneapolis, Minnesota (USA), 9–12 October 2011, Geological Society of America, Paper No. 231-10, https://gsa.confex.com/gsa/2011AM/webprogram/Paper192992.html (last access: 10 June 2022), 2011.
Hammer, Ø., Harper, D. A. T., and Ryan, P. D.: PAST: Paleontological Statistics Software Package for education and data analysis, Palaeontol. Electron., 4, https://palaeo-electronica.org/2001_1/past/past.pdf (last access: 7 September 2021), 2001.
Hayek, L.-A. C. and Buzas, M. A.: On the proper and efficient use of diversity measures with individual field samples, J. Foramin. Res., 43, 305–313, https://doi.org/10.2113/gsjfr.43.3.305, 2013.
Hayward, B. W., Le Coze, F., Vachard, D., and Gross, O.: World Foraminifera database, World Register of Marine Species, https://doi.org/10.14284/305, 2022.
Higgins, M. D.: Greek Islands, Geology, in: Encyclopedia of Islands, edited by: Gillespie, R. and Clague, D., University of California Press, Berkeley, 392–396, https://doi.org/10.1525/9780520943728-092, 2009.
Hottinger, L., Halicz, E., and Reiss, Z.: Recent foraminiferida from the Gulf of Aqaba, Red Sea. Razred za naravoslovne vede, classis IV, historia naturalis, opera 33, Slovenska Akademia, Ljubljana, 179 pp., ISBN 8671310760, 1993.
Koukousioura, O., Dimiza, M. D., and Triantaphyllou, M. V.: Alien foraminifers from Greek coastal areas (Aegean Sea, Eastern Mediterranean), Medit. Mar. Sci., 11, 155–172, https://doi.org/10.12681/mms.98, 2010.
Koukousioura, O., Dimiza, M. D., Triantaphyllou, M. V., and Hallock, P.: Living benthic foraminifera as an environmental proxy in coastal ecosystems: A case study from the Aegean Sea (Greece, NE Mediterranean), J. Marine Syst., 88, 489–501, https://doi.org/10.1016/j.jmarsys.2011.06.004, 2011.
Langer, M. R.: Recent Epiphytic Foraminifera from Vulcano (Mediterranean Sea) – Rev. Paléobio, Vol. Spéc. 2, BENTHOS'86, Third International Symposium on Benthic Foraminifera, 22–28 September 1986, Muséum d'Histoire Naturelle, Genève, Switzerland, 827–832, 1988.
Langer, M. R.: Epiphytic foraminifera, Mar. Micropaleontol., 20, 235–265, https://doi.org/10.1016/0377-8398(93)90035-V, 1993.
Langer, M. R. and Lipps, J. H.: Foraminiferal distribution and diversity, Madang Reef and Lagoon, Papua New Guinea, Coral Reefs, 22, 143–154, https://doi.org/10.1007/s00338-003-0298-1, 2003.
Langer, M. R. and Mouanga, G. H.: Invasion of amphisteginid foraminifera in the Adriatic Sea, Biol. Invasions, 18, 1335–1349, https://doi.org/10.1007/s10530-016-1070-0, 2016.
Langer, M. R., Weinmann, A. E., Lötters, S., and Rödder, D.: “Strangers” in paradise: modeling the biogeographic range expansion of the foraminiferal Amphistegina in the Mediterranean Sea, J. Foramin. Res., 42, 234–244, https://doi.org/10.2113/gsjfr.42.3.234, 2012.
Larsen, A. R.: Studies of Recent Amphistegina, Taxonomy and some Ecological Aspects, Israel J. Earth Sci., 25, 1–26, 1976.
Maragoudakis, N.: The Neogene foraminifera of Corfu Island, Bulletin of the Geological Society of Greece, 4, 65–68, 1961.
Maragoudakis, N.: Geology and micropaleontology of southern Corfu, Geological and Geophysical Research, 12, 1–132, 1967 (in Greek).
Massi, D., Titone, A., Gristina, M., Garofolo, G., Lauria, V., Micalizzi, R., Sinacori, G., and Fiorentino, F. Characterization and biogeographic affinity of megazoobenthos in the Central Mediterranean Sea, Mar. Ecol., 42, e12627, https://doi.org/10.1111/maec.12627, 2021.
Mateu-Vicens, G., Box, A., Deudero, S., and Rodriguez, B.: Comparative analysis of epiphytic foraminifera in sediments colonized by seagrass Posidonia oceanica and invasive macroalgae Caulerpa spp., J. Foramin. Res., 40, 134–147, https://doi.org/10.2113/gsjfr.40.2.134, 2010.
Mateu-Vicens, G., Khokhlova, A., and Sebastian-Pastor, T.: Epiphytic foraminiferal indices as bioindicators in Mediterranean seagrass meadows, J. Foramin. Res., 44, 325–339, https://doi.org/10.2113/gsjfr.44.3.325, 2014.
Meriç, E., Avşar, N., Nazik, A., Yokeş, M. B., and Dinçer, F.: A Review of Benthic Foraminifers and Ostracodes of the Antalya Coast, Micropaleontology, 54, 199–240, https://www.micropress.org/microaccess/micropaleontology/issue-251/article-1581 (last access: 13 July 2023), 2008.
Meriç, E., Avşar, N., Yokeş, M. B., and Dinçer, F.: Atlas of recent benthic foraminifera from Turkey, Micropaleontology, 60, 211–398, https://www.micropress.org/microaccess/micropaleontology/issue-309 (last access: 27 July 2021), 2014.
Milker, Y. and Schmiedl, G.: A taxonomic guide to modern benthic shelf foraminifera of the western Mediterranean Sea, Palaeontol. Electron., 15, 15.2.16A, https://doi.org/10.26879/271, 2012.
Mouanga, G. H.: Impact and range extension of invasive foraminifera in the NW Mediterranean Sea: implications for diversity and ecosystem functioning, PhD thesis, University of Bonn (Germany), 169 pp., https://hdl.handle.net/20.500.11811/7491 (last access: 14 August 2020), 2018.
Mouanga, G. H. and Langer, M. R.: At the front of expanding ranges: Shifting community structures at amphisteginid species range margins in the Mediterranean Sea, Neues Jahrb. Geol. P.-A., 271, 141–150, https://doi.org/10.1127/0077-7749/2014/0381, 2014.
Murray, J. W.: Distribution and Ecology of Living Benthic Foraminiferids, Crane, Russak & Co., New York, 274 pp., ISBN 0435624318, 1973.
Murray, J. W.: Ecology and Applications of Benthic Foraminifera, Cambridge University Press, Cambridge, 426 pp., https://doi.org/10.1017/CBO9780511535529, 2006.
Murray, J. W. and Bowser, S. S.: Mortality, protoplasm decay rate and reliability of staining techniques to recognize “living” foraminifera: a review, J. Foramin. Res., 30, 66–70, https://doi.org/10.2113/0300066, 2000.
Parent, B., Barras, C., Bicchi, E., Charrieau, L. M., Choquel, C., Bénéteau, E., Maillet, G. M., and Jorissen, F. J.: Comparison of four foraminiferal biotic indices assessing the environmental quality of coastal Mediterranean soft bottoms, Water, 13, 3193, https://doi.org/10.3390/w13223193, 2021.
Pisano, A., Marullo, S., Artale, V., Falcini, F., Yang, C., Leonelli, F. E., Santoleri, R., and Buongiorno Nardelli, B.: New evidence of Mediterranean climate change and variability from sea surface temperature observations, Remote Sens., 12, 132, https://doi.org/10.3390/rs12010132, 2020.
Rögl, F., Antl-Weiser, W., Brandstätter, F., Dermitzakis, M. D., Papesch, W., Piller, W. E., Schultz, O., Symeonidis, N. K., Triantaphyllou, M. V., and Tsarpalis, V.: Late Pleistocene marine circles in Southern Corfu, Annales Géologiques des Pays Helléniques, 37, 663–767, 1997.
Sariaslan, N. and Langer, M. R.: Atypical, high-diversity assemblages of foraminifera in a mangrove estuary in northern Brazil, Biogeosciences, 18, 4073–4090, https://doi.org/10.5194/bg-18-4073-2021, 2021.
Shannon, C. E.: A mathematical theory of communication, Bell Syst. Tech. J., 27, 379–423, https://doi.org/10.1002/j.1538-7305.1948.tb01338.x, 1948.
Stulpinaite, R., Hyams-Kaphzan, O., and Langer, M. R.: Alien and cryptogenic Foraminifera in the Mediterranean Sea: A revision of taxa as part of the EU 2020 Marine Strategy Framework Directive, Mediterr. Mar. Sci., 21, 719–758, https://doi.org/10.12681/mms.24673, 2020.
Triantaphyllou, M. and Dimiza, M.: Amphistegina lobifera in Zakynthos island, Ionian Sea, in: New Mediterranean Marine biodiversity records (June 2013), edited by: Siokou, I., Ates, A. S., Ayas, D., Ben Souissi, J., Chatterjee, T., Dimiza, M., Durgham, H., Dogrammatzi, K., Erguden, D., Gerakaris, V., Grego, M., Issaris, Y., Kadis, K., Katagan, T., Kapiris, K., Katsanevakis, S., Kerckhof, F., Papastergiadou, E., Pesic, V., Polychronidis, L., Rifi, M., Salomidi, M., Sezgin, M., Triantaphyllou, M., Tsiamis, K., Turan, C., Tziortzis, I., d'Udekem d'Acoz, C., Yaglioglu, D., Zaouali, J., and Zenetos, A., Mediterr. Mar. Sci., 14, 242–243, https://doi.org/10.12681/mms.450, 2013.
Triantaphyllou, M. V., Drinia, H., and Dermitzakis, M. D.: Quantitative micropaleontological analysis and paleoenvironmental interpretation of Southern Kerkyra Pliocene deposits, Géologie Mediterranéenne, 22, 111–123, https://doi.org/10.3406/geolm.1995.1573, 1995.
Triantaphyllou, M. V., Tsourou, T., Koukousioura, O., and Dermitzakis, M. D.: Foraminiferal and ostracod ecological patterns in coastal environments of SE Andros Island (Middle Aegean Sea, Greece), Rev. Micropaleontol., 48, 279–302, https://doi.org/10.1016/j.revmic.2005.09.003, 2005.
Triantaphyllou, M. V., Koukousioura O., and Dimiza, M. D.: The presence of the Indo-Pacific symbiont-bearing foraminifer Amphistegina lobifera in Greek coastal ecosystems (Aegean Sea, Eastern Mediterranean), Medit. Mar. Sci., 10, 73–85, https://doi.org/10.12681/mms.111, 2009.
Triantaphyllou, M. V., Dimiza, M. D., Koukousioura, O., and Hallock, P.: observations on the life cycle of the symbiont-bearing foraminifer Amphistegina lobifera Larsen, an invasive species in coastal ecosystems of the Aegean Sea (Greece, E. Mediterranean), J. Foramin. Res., 42, 143–150, https://doi.org/10.2113/gsjfr.42.2.143, 2012.
Tserolas, P., Mpotziolis, C., Maravelis, A., and Zelilidis, A.: Preliminary geochemical and sedimentological analysis In NW Corfu. The Miocene sediments In Agios Georgios Pagon, Bulletin of the Geological Society of Greece, 50, 402–412, https://doi.org/10.12681/bgsg.11741, 2016.
Tsiamis, K., Azzurro, E., Bariche, M., Çinar, M. E., Crocetta, F., De Clerk, O., Galil, B., Gómez, F., Hoffman, R., Jensen, K. R., Kamburska, L., Langeneck, J., Langer, M. R., Levitt-Barmats, Y., Lezzi, M., Marchini, A., Occhipinti-Ambrogi, A., Ojaveer, H., Piraino, S., Shenkar, N., Yonkova, M., Zenetos, A., Žuljević, A., and Cardoso, A. C.: Prioritizing marine invase alien species in the European Union through horizon scanning, Aquat. Conserv., 30, 794–845, https://doi.org/10.1002/aqc.3267, 2020.
Vohník, M.: Bioerosion and fungal colonization of the invasive foraminiferan Amphistegina lobifera in a Mediterranean seagrass meadow, Biogeosciences, 18, 2777–2790, https://doi.org/10.5194/bg-18-2777-2021, 2021.
Weinmann, A., Koukousioura, O., Triantaphyllou, M., and Langer, M.: Datasets to Weinmann et al.: Spatial distribution and diversity of benthic shallow-water foraminifera from Corfu Island (Greece, Ionian Sea): An island at the range front of an invasive species, NHMW Data Repository [data set], https://doi.org/10.57756/9uhty3, 2022.
Weinmann, A. E. and Langer, M. R.: Diverse thermotolerant assemblages of benthic foraminiferal biotas from tropical tide and rock pools of eastern Africa, Rev. Micropaleontol., 60, 511–523, https://doi.org/10.1016/j.revmic.2017.09.002, 2017.
Weinmann, A. E., Rödder, D., Lötters, S., and Langer, M. R.: Traveling through time: The past, present and future biogeographic range of the invasive foraminifera Amphistegina spp. in the Mediterranean Sea, Mar. Micropaleontol., 105, 30–39, https://doi.org/10.1016/j.marmicro.2013.10.002, 2013.
Weinmann, A. E., Goldstein, S. T., Triantaphyllou, M. V., and Langer, M. R.: Effects of sampling site, season, and substrate on foraminiferal assemblages grown from propagule banks from lagoon sediments of Corfu Island (Greece, Ionian Sea), PLoS One, 14, e0219015, https://doi.org/10.1371/journal.pone.0219015, 2019.
Yokeş, M. B., Meriç, E., and Avşar, N.: On the Presence of Alien Foraminifera Amphistegina lobifera Larsen on the coasts of the Maltese Islands, Aquat. Invasions, 2, 439–441, https://doi.org/10.3391/ai.2007.2.4.15, 2007.
Yokeş, M. B., Meriç, E., Avşar, N., Öncel, M. S., Eryilmaz, M., and Barut, İ.: The expanded population of Amphistegina lobifera at Üç Adalar and Beş Adalar (Antalya, Turkey), Mar. Biodivers. Rec., 7, e52, https://doi.org/10.1017/S175526721400044X, 2014.