the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Water-mediated changes in plant–plant and biological soil crust–plant interactions in a temperate forest ecosystem
Clara Pissolito
Irene A. Garibotti
Santiago A. Varela
Verónica Arana
Marina Gonzalez-Polo
Paula Marchelli
Octavio Bruzzone
In the quest to understand how biotic interactions respond to climate change, one area that remains poorly explored is how interactions involving organisms other than vascular plants will respond. However the interactions between plants and biological soil crusts (BSCs) are relevant in many ecosystems and they will likely respond uniquely to climate change. Simultaneous considerations of both plant–plant and plant–BSC interactions may substantially improve our understanding of this topic. The aim of this study is to assess whether water availability differentially affects the biotic effects of BSCs and pioneer shrubs on the early life-history stage of tree seedling growth. We conducted a greenhouse factorial experiment with soil surface cover (bare soil, soil covered by a creeping shrub and BSC covered soil) and water regime (control and drought) as factors. We monitored Nothofagus pumilio (a native tree species of ecological and economic relevance) seedling water status and growth as well as changes in soil water content and soil properties. The shrub cover had a positive effect on soil water conservation and on the water balance of seedlings under water stress. However, its effect was negative for seedling growth under both water conditions. The BSC also contributed to soil water conservation and apparently added nutrients to the soil. The net effect of the BSC on seedling growth was negative under full-watering conditions but positive under water stress conditions. This result highlights how the studied biotic interactions, and especially interactions involving BSCs, depend on changes in water availability.
- Article
(2028 KB) - Full-text XML
- BibTeX
- EndNote
Interest in studying the impacts of biotic interactions and environmental change is growing since it is now clear that biotic interactions play a central role in how plant communities may respond to global change (Tylianakis et al., 2008). For instance, plants influence their surrounding environment, changing microclimatic conditions by altering radiation, air and soil temperatures (Callaway and Walker, 1997). These environmental changes and their impact on other organisms in turn interact with changes in macroclimatic conditions. Therefore, the future climate plants will experience will depend not only on global change scenarios but also on neighboring organisms.
For the last 25 years much of the research focusing on how biotic interactions change in relation to varying environmental factors has revolved around Bertness and Callaway's stress gradient hypothesis (SGH; Bertness and Callaway, 1994), which predicts an increase in positive interactions with increasing environmental stress. Since its formulation, copious evidence has been found in support of the SGH (He et al., 2013). However, evidence has also been found indicating that the SGH is not universal (Maestre et al., 2005; Bowker et al., 2010). Recent reformulations of the SGH also describe a hump-shaped response of positive interactions to environmental stress, with interactions shifting back to neutral or negative at the extremes of gradients, particularly if aridity is involved (Bowker et al., 2010; Butterfield et al., 2016). Also, several studies indicate that shrubs are often the life form most likely to have a net positive effect on other organisms since they change microsites beneath their canopies by improving physical conditions (Pugnaire and Luque, 2001), while they have softer competitive effects compared to other life forms such as grasses (Gómez-Aparicio, 2009; Butterfield and Briggs, 2011). To date, the SGH remains a broad and useful theoretical frame for understanding changes in biotic interactions along environmental gradients and predicting how these interactions could be affected by climate change; however, further studies are needed (Dullinger et al., 2007).
One area that has received less attention is how biotic interactions involving other organisms besides vascular plants respond to change (but see Maestre et al., 2010; Doxford et al., 2012). Organisms such as mosses and lichens are also involved in biotic interactions within plant communities but have morphological and physiological traits and responses to environmental changes that can be very different from those of vascular plants. For example, many of these organisms are poikilohydric (i.e., their water content varies passively according to water availability) meaning that their response to drought are unlikely to resemble those of vascular plants.
In some situations, lichens, bryophytes, algae and cyanobacteria can form a cohesive structure on the soil surface denominated biological soil crust (BSC; Belnap et al., 2016). The BSC influences environmental and biotic conditions, including changes in temperature, moisture, water runoff, nutrient availability and microbial activity (Belnap, 2003), thus affecting vascular plants. The positive impacts of BSC on vascular plants include increased seed trapping (Zhang et al., 2016) and augments in the availability of water and nutrients (Pendleton et al., 2003; Breen and Lévesque, 2006; Langhans et al., 2009). Negative effects are associated with physical barriers or specific exudates from BSCs that prevent seed entrapment and germination (Serpe et al., 2006; Langhans et al., 2009), direct competition for water and nutrients (Thiet et al., 2014) and reduced rain infiltration (Kidron, 2014; Xiao and Hu, 2017; Shi et al., 2018). Neutral or mixed positive and negative effects have also been reported (Zaady et al., 1997; Su et al., 2007; Funk et al., 2014). The BSC's effects on vascular plants varies depending on plant traits (Zaady et al., 1997; Zhang and Belnap, 2015), the morphology and chemical characteristics of the BSC species (Serpe et al., 2006; Langhans et al., 2009) and environmental conditions (Su et al., 2007; Langhans et al., 2009). Previous studies have demonstrated the ecological relevance of BSCs in arid and semiarid areas (Weber et al., 2016) and in other harsh and sparsely vegetated environments such as polar deserts or high mountain communities (Gold et al., 2001; Breen and Lévesque, 2006; Schmidt et al., 2008; Garibotti et al., 2011; Dvorský, 2014). However, studies examining how their impacts change along gradients are rare (but see Maestre et al., 2010; Xiao and Hu, 2017). Other noticeable knowledge gaps include their roles in temperate forest systems, although they have also been described in this habitat (Büdel, 2001), and in South America (Weber et al., 2016).
The aim of this study was to assess whether water availability differentially affects the biotic effects of BSCs and pioneer shrubs on the early plant life-history stage of tree seedling growth. This study uses Nothofagus pumilio (Poepp. et Endl.) Krasser seedlings as a focal organism, since this species is one of the most widely distributed trees in the Patagonian forests. Furthermore, N. pumilio has great ecological and economic relevance; it is the species that commonly forms the altitudinal tree line, protecting the heads of watersheds, and at lower elevation it produces valuable timber and summer grazing grounds. Patagonian forests are already facing a desiccating trend in climatic conditions: decrease in precipitation and increase in temperature, which is expected to intensify according to climate change scenarios, especially toward the north of their distribution (Barros et al., 2014). This can have important ecological consequences since N. pumilio forest dynamics and establishment patterns are greatly shaped by climatic trends (Rodríguez-Catón et al., 2016; Srur et al., 2016). We hypothesized that the cover of shrubs and the BSC have a facilitative effect on N. pumilio seedlings under water shortage since they modulate abiotic stress by increasing soil water availability. In addition, we hypothesized that the BSC would have an additional positive effect by increasing soil fertility while shrubs compete with seedlings for nutrient resources. Exploring this topic could improve our understanding of biotic interactions and help predict the ecological responses of these forests to climate change.
2.1 Study system
The temperate forests of Patagonia occupy a narrow strip on both flanks of the Patagonian Andes, spanning more than 1000 km from north to south at the southern end of South America. Temperature and precipitation in this region are very variable, both spatially (Lenaerts et al., 2014) and temporally (Garreaud et al., 2013). In the northeast of Andean Patagonia, where we conducted the study (Fig. 1a), the mean annual temperature and precipitation have been modeled as 9–10 ∘C and 800–1000 mm yr−1 respectively, with the bulk of the annual precipitation occurring in autumn and winter (Bianchi et al., 2016). Superimposed on the historic natural variation, climate change in Patagonia translates to drier conditions (temperature rise and precipitation decrease) which have already been observed and are predicted to persist (Barros et al., 2014). We selected Nothofagus pumilio as a focal organism, as it is a native deciduous tree forming pure or mixed stands over a great variety of environmental conditions along and across the Patagonian Andes. Creeping shrubs and BSCs are common cover types in areas where N. pumilio forests have been altered, and also in forest margins and above the forest tree line (Fig. 1b; Ferreyra et al., 2006), and as such they represent organisms with which recruiting N. pumilio individuals would interact. As a creeping shrub we selected Empetrum rubrum (Vahl ex Willd.) which is widely distributed in the subantarctic islands, Fuegian Archipelago and all along the Patagonian Andes, forming dense woody carpets 2–12 cm tall that grow trailing the soil surface (Ferreyra et al., 2006).
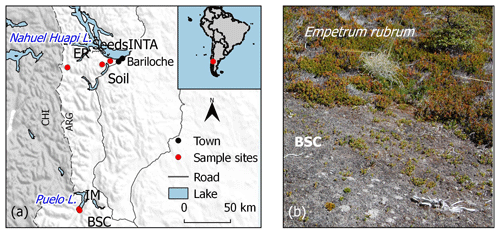
Figure 1Location of the experimental station (INTA) and sampling sites of soil, inert (IM) and plant materials (a); mosaic of Empetrum rubrum and biological soil crust (BSC) in a natural open area within a Nothofagus pumilio forest (b).
The BSC used in this experiment was dominated by the lichens Cladonia pocillum, C. subulata, C. cyathomorpha, Diploschistes muscorum and Placynthiella uliginosa; the moss Racomitrium lanuginosum; cyanobacteria, and to a lesser extent the lichen Peltigera polydactylon which has N-fixing photobionts. Nomenclature follows Calvelo and Liberatore (2002) for lichens and Brummit and Powell (1992) for bryophytes.
2.2 Experimental design
The experiment was conducted under greenhouse conditions during a 38-day period starting 25 February 2014 (austral summer), in an experimental station of INTA (National Institute of Agricultural Technology) in Bariloche, northern Patagonia (Fig. 1a). To test our hypotheses, we compared the response of N. pumilio seedlings to water deficit under three contrasting soil cover types. The design included two factors: soil cover type with three levels (bare soil, soil covered by the BSC and soil covered by the shrub Empetrum rubrum) and watering regime with two levels (drought and control) in a 3×2 factorial design. There were three replicates per level of each factor; thus the total number of microcosms was 18. The greenhouse was kept well ventilated during the study; maximum and minimum daily temperatures were 20 ∘C (±3) and 7 ∘C (±4) respectively, registered with a wireless thermometer (WHO100; Sinometer Instruments, Shenzhen, China).
2.2.1 Microcosm construction
Each microcosm consisted of a 10 L polypropylene cylindrical container (22.5 cm diameter, 28.5 cm height) filled in the same way for all treatments, differing only in the top layer according to the soil cover type factor. To ensure proper drainage, containers were filled in the bottom with a 7 cm deep layer of inert materials (rocks, 5 cm diameter and 5 cm deep; gravel <2 cm diameter and 2 cm deep; and interspaces filled with sand) obtained from a river bank, and holes were drilled at the base of the containers. The middle part of the containers had a layer of 15 cm of natural N. pumilio forest soil. Natural soils of N. pumilio forests at this latitude are andosols, which are derived from volcanic materials (Etchevehere, 1972; del Valle, 1998). The soil used had a loam texture and was thoroughly mixed before filling the microcosms in order to homogenize it and minimize possible differences among replicates. To ensure homogeneity in the content of the containers, the height of each layer was measured from outside with a ruler. The containers were topped with live patches of either E. rubrum or BSC, while for the bare soil treatment the forest soil was left as the top layer. The E. rubrum patches were collected from an area where this species forms a monospecific 5 cm tall cover of the N. pumilio forest floor by cutting cores (15 cm in diameter and 10 cm deep) with a shovel, and the patches were transported in closed plastic bags to the experimental station. The bottom of the cores was then scraped by hand to remove soil, and the chunks of shrub carpet were placed with bare roots in trays with running water for 48 h. This washing procedure removed the remaining soil from the roots and was carried out to ensure that no extra soil was added to the microcosms. This procedure also has been proven to wash out rooting inhibitors and to enhance rooting of other native species (Rolón et al., 2013). Finally, the chunks of E. rubrum with clean bare roots were planted on top of the containers until the complete surface was covered. The BSC was obtained by cutting square patches (10 cm×10 cm, 3 cm deep) from an open area with homogeneous BSC cover and they were placed in empty containers for transportation. At the experimental station we arranged the patches on top of the filled containers and trimmed the edges to obtain a complete cover.
The microcosms were constructed in October 2013 and kept outside of the greenhouse at the experimental station until the beginning of the experiment in order to detect possible death of E. rubrum or changes in BSC. No death or change was observed during the 3.5-month period before the start of the experiment. The rocks, gravel and BSC patches were collected in the Lago Puelo National Park, while the E. rubrum patches and forest soil were collected in the Nahuel Huapi National Park (42∘11′ S, 71∘41′ W and 41∘09′ S, 71∘18′ W, respectively), in areas with very low human impact (Fig. 1).
2.2.2 Seedling production
Nothofagus pumilio seedlings used in the experiment were grown in a nursery at INTA from seeds collected by the authors in March 2011, from a mature N. pumilio stand in Nahuel Huapi National Park (Fig. 1). Seeds were collected at the time of their natural dispersal from several parent trees, pooled and stored in paper bags at 4 ∘C until used. Prior to sowing, seeds were stratified at 4 ∘C for 60 d on cotton moistened with 1 % fungicide (Vitavax®FLO, Lujan Agrícola, Mendoza, Argentina) to break dormancy. Three and a half months following emergence, six seedlings (15–20 cm tall with 10 true leaves and no yellowing or signs of herbivory) were transplanted into each container to a depth of 10 cm and allowed to acclimatize to the microcosm conditions for two weeks prior to the initiation of the experiment.
2.2.3 Watering regime
The control treatment was watered to saturation three times a week throughout the experiment and allowed to drain through holes at the bottom of the microcosms. The drought treatment was watered to saturation (i.e., pot capacity sensu Passioura, 2006) at the beginning of the experiment, and thereafter watered once a week with a variable amount of water determined as 50 % of the weight loss by evapotranspiration since the previous watering; so that a progressively smaller amount of water was used each time in order to achieve a gradual drop in the substrate moisture content. Watering of the drought treatment was suspended during the last two weeks of the experiment.
2.3 Variables measured
The volumetric water content (VWC; % v/v) of soil was measured in all microcosms three times a week for 38 d using a time domain reflectometer (HydroSense with 12 cm rod sensors, CS620 Campbell Scientific Inc., Logan, Utah, USA). Midday stomatal conductance (gs; mmol H2O m−2 s−1) was measured 3 d a week in fully expanded leaves of six seedlings per treatment, also for the 38-day experiment, with a leaf porometer (SC-1, Decagon Devices Inc., Pullman, Washington, USA). All VWC and gs measurements were performed between 09:00 and 11:00 when the greenhouse was in full sunlight. The water status of seedlings was monitored by measuring pre-dawn water potential (Ψpd; MPa) in the terminal shoots of three seedlings per treatment with a pressure chamber (PMS 1000, PMS Instrument Company, Corvallis, Oregon, USA). Measurements of Ψpd were performed only three times when a substantial drop in VWC was detected (on days 14, 31 and 38 since the experiment onset) as it was a destructive procedure. At the end of the experiment two seedlings from each container were harvested and dried at 65 ∘C to constant weight to determine the aerial and underground biomass (g). Soil was sampled (0–3 cm depth from each cover type) before starting the experiment (n=1) and sampled from each treatment at the end of the experiment (n=3). Soil samples were sieved through a 2 mm mesh and air dried for 1 week to measure the pH in water and the electrical conductivity (1 : 2.5 and 1 : 5 soil–water ratio, respectively). Phosphorous (P) was extracted in 0.5 M NaHCO3 (1 : 20, soil–solution ratio) and determined by the molybdate–ascorbic acid method (Kuo, 1996). Total carbon (C) and nitrogen (N) were determined by dry combustion (Thermo Electron, FlashEA 1112, Rodano, Milan, Italy). Inorganic N was evaluated using 2 N KCl extracts, and then ammonium was determined by the indophenol-blue method and nitrate by copperized Cd reduction (Keeney and Nelson, 1982).
2.4 Data analysis
Differences in final soil properties, seedling dry weights and S∕Rs (shoot-to-root ratios) among treatments, including their combinations, were compared with two-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests with type of soil cover and watering conditions as factors. The significance of main factors or their interaction was determined according to α=0.05. If the main factors were significant and the interaction between factors was not significant, differences among treatment combinations were determined according to a Bonferroni corrected α=0.003 (; where and k = number of treatment combinations; in our case k=6 ). One-way repeated-measures ANOVAs were used to test the effect of the watering regime on seedling water potential. This variable was transformed by applying the natural logarithm of the absolute value in order to achieve normality and homogeneity of variances.
To summarize the major trends in gs and VWC, we applied a smoothing-fitting method based on locally weighted polynomial regressions (LOESS), using gs or VWC as a dependent variable, and time since the experiment onset as an independent variable. This method is used to provide a graphical summary of complex relationships (Jacoby, 2000). We selected the best smoothing parameters by examining plots of the fit residuals vs. the predictor variable. To evaluate the relationship between gs and soil VWC in each cover type under restricted watering we performed a linear regression with a stepwise series of autoregressive integrated moving average models (ARIMA models; Box and Jenkins, 1976) for controlling the autocorrelation of errors. The model with lowest AIC was selected as the best balancing fit and complexity. If the model with external regressors is selected it means that the external variable might have an effect on the values of the response variable; otherwise all the observed variation might be random internal variation of the time series. All statistical analyses were performed with InfoStat (Di Rienzo et al., 2017), except the ARIMA models, which were performed in R using the “arima” function of the “tseries” library.
To quantify the outcome of interactions between N. pumilio seedlings and E. rubrum and the BSC, we calculated the relative interaction index (RII) which measures the net balance of biotic interactions on plant biomass (Armas et al., 2004). RII is expressed as , where Bw is the dry weight of the target species growing with an accompanying organism and Bo is the weight of the target species in absence of interactions. The RII of a target plant ranges from −1 for a plant completely outcompeted by another plant to +1 for a plant facilitated by another plant.
3.1 Soil conditions under different surface covers
Throughout the experiment, soil water content was relatively stable in the control of all three substrates, with average values of 13.6±1.2 %, 11.6±1.8 % and 11.3±1.5 % (mean ± SE), for E. rubrum, BSC and bare soil treatments, respectively (Fig. 2a, c, e). Fluctuations corresponded to the watering events. Under reduced watering, soil VWC rapidly dropped below 5 % v/v after 10 d for bare soil, while persisting for about 20 d above 5 % v/v for crusted soils and was above 10 % v/v for the soils covered by E. rubrum (Fig. 2b, d, f). The bare soil and BSC substrate types showed a considerable drop in the VWC when watering was suspended after the 25th day of the experiment (Fig. 2d, f).
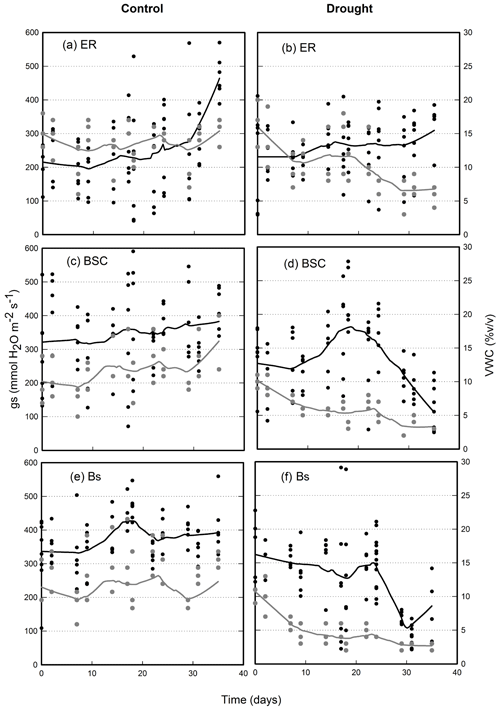
Figure 2Volumetric water content of soil (VWC, in gray) and stomatal conductance of Nothofagus pumilio seedlings (gs, in black) grown in different treatments of soil cover and under conditions of full and restricted watering (control and drought, respectively). Crosses are mean values for each measured date and lines are locally weighted polynomial regressions (LOESS) performed on all values for each date. ER, soil covered by Empetrum rubrum; BSC, soil covered by biological soil crust; and Bs, bare soil.
Table 1Properties of bare soil and soils covered by biological soil crust and Empetrum rubrum before (initial) and after applying a full watering (control) and a partial soil drying regimen (drought) during a 38-day period. Values are means ±1 standard error (n=3). Differences among final conditions were determined using two-way ANOVA followed by Bonferroni post hoc comparisons. Bold values represent significant interaction between substrate type and watering level (p≤0.05). Underlines and italics represent significance of simple main effects (watering level and substrate type, respectively) (p≤0.05). Within each column and substrate cover type, values sharing a letter (a, b or c) are not statistically different from each other (Bonferroni-corrected p≤0.003).
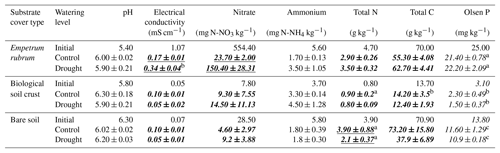
In the initial soil samples, we found differences in most chemical and physical variables measured among soils covered by BSC, bare soils, or soils covered by E. rubrum (Table 1). At the end of the experiment, differences in soil properties between the control and drought conditions were non-significant for soils covered by BSC, but there was a significant effect (p<0.05) of watering conditions on the nitrate content and electrical conductivity of the E. rubrum covered soils and on the total nitrogen content of bare soils. One interesting yet not significant trend is the higher final concentration of ammonium observed under drought compared to the control for the soil covered by E. rubrum (Table 1). It is also worth mentioning the slight increase in nitrate and ammonium concentrations in the BSC covered soils at the end of the experiment in comparison to the initial concentration, while the E. rubrum and bare soil treatments showed an opposing pattern of decreasing concentrations in both the control and drought conditions at the end of the experiment (Table 1).
3.2 Seedlings' performance and growth
The interaction index (RII) was negative for seedlings grown with E. rubrum under both watering conditions (Fig. 5c). A positive value of RII was obtained for seedlings growing in soils covered by BSC under restricted watering, but a negative value under full watering was observed (Fig. 5c).
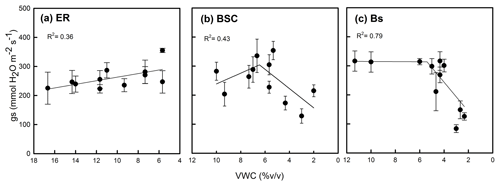
Figure 3Relationship between volumetric water content (VWC) of soil and stomatal conductance (gs) of Nothofagus pumilio seedlings grown in different treatments of soil cover under conditions of restricted watering. ER, soil covered by Empetrum rubrum; BSC, soil covered by biological soil crust; and Bs, bare soil. Note the different range of the x axis for panel (a). Values are the average per measuring date, and fitted regressions are linear or piecewise linear. Statistical analyses of the influence of soil VWC on seedling gs are detailed in Table 2.
The stomatal conductance (gs) of seedlings growing in soils covered by E. rubrum did not decrease due to limited watering, but on the contrary gs slightly increased in both watering conditions as the experiment progressed (Fig. 2a, b). Under restricted watering, there was a negative relationship between seedlings' gs and soil VWC (Fig. 3a), but the outcome of the ARIMA model indicated no explanatory effect of VWC on gs (Table 2). In addition, the Ψpd of seedlings did not differ significantly between watering conditions and did not vary significantly during the whole experiment (Fig. 4a). At the end of the experiment, seedlings grown in soils covered by E. rubrum had significantly lower total biomass in comparison to those grown in bare soil, which was mainly due to a reduced development of roots (Fig. 5a, b).
Table 2Results of the best fit autoregressive integrated moving average (ARIMA) models of the variation in stomatal conductance of Nothofagus pumilio seedlings grown under water restriction in three soil cover conditions, using soil volumetric water content as the explicative variable. The parameters in the model are AR, autoregressive coefficient; I, integrated error term; and MA moving average term. X is the linear regression coefficient of the explicative variable.
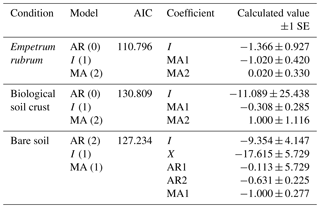
In soils covered by BSC, water-restricted seedlings had higher values of gs at intermediate conditions of soil VWC, although soil VWC did not explain the tendency of gs as drought conditions intensified (Figs. 2d and 3b, Table 2). Seedlings subjected to water stress showed a significant decrease in their Ψpd as drought intensified (Fig. 4b). At the end of the experiment, seedling biomass was slightly greater under stress than under the well-watered condition, but differences in biomass were not statistically significant (Fig. 5a, b).
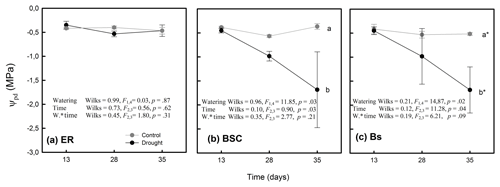
Figure 4Predawn water potential (Ψpd) values for Nothofagus pumilio seedlings grown in different soil cover types and under conditions of full and restricted watering (control and drought, respectively). ER, soil covered by Empetrum rubrum; BSC, soil covered by biological soil crust; and Bs, bare soil. Values are the means of three replicates per treatment ± standard error. Significance values (p) are from one-way, repeated-measures ANOVAs testing the effects of watering regime over time. Wilks indicates the Wilks' lambda value.
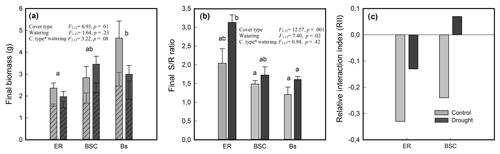
Figure 5(a) Final biomass (dry weight) of shoot (striped pattern) and root (plain colored), (b) shoot-to-root ratio (S∕R) and (c) relative interaction index (RII) of Nothofagus pumilio seedlings grown in different cover types and under conditions of full and restricted watering (control and drought, respectively). ER, soil covered by Empetrum rubrum; BSC, soil covered by biological soil crust; and Bs, bare soil. Biomass and S∕R ratio values are means of 3 replicates ± standard error. Significance values (p) are from two-way ANOVAs testing effects of soil cover type and watering regime. If main effects or interactions were significant individual comparisons are not shown. Different lower-case letters indicate significantly different values by a posteriori tests at (p≤0.05) for interaction, or (p≤0.003) for main effects.
In seedlings growing in bare soils, gs slightly increased in the fully watered condition as the experiment progressed, whereas in seedlings subjected to low watering conditions gs abruptly diminished when the soil VWC fell below 5.5 % v/v (Figs. 2e, f and 3c). In the water-restricted treatment, the soil VWC had an explanatory effect on gs and this tendency was strongly time dependent (Table 2). The Ψpd was significantly depressed in the water stressed seedlings relative to control seedlings (Fig. 4c). Seedlings growth was not significantly different between the reduced and full-watering conditions (Fig. 5a).
In line with predictions of the stress gradient hypothesis (SGH), we found that the effects of neighbors on seedlings tended to be more positive under stress. Partially supporting our expectations, we found a positive effect of the biological soil crust (BSC) on soil water and nutrient resources and a positive effect of shrub cover on soil moisture, but the effects on seedling growth were more complex than anticipated. The BSC had a positive effect on Nothofagus pumilio seedlings growth under stress conditions, but Empetrum rubrum had a negative effect on growth in all water conditions and a positive effect on seedling water status under water shortage. These findings indicate that water availability influences the outcome of the biotic interactions between a shrub and tree seedlings and between BSC and tree seedlings in a temperate forest ecosystem.
4.1 Potential effects of Empetrum rubrum on the tree seedlings
Previous studies in different temperate forests of Patagonia have shown that shrubs have an overall positive effect on the recruitment of different tree species in a variety of habitats (Raffaele and Veblen, 1998; Kitzberger et al., 2000; Henríquez and Lusk, 2005; Nuñez et al., 2009; Bustamante-Sánchez et al., 2011). This contrasts with the negative interaction index (RII) values we observed in this study, which indicate a net negative effect of the shrub on N. pumilio seedlings. This negative effect of the shrub could possibly be due to the greater biomass of the shrub compared to the tree seedlings at this stage. Another possibility is interference effects, which have been widely documented for other ericaceous shrubs (Nilsson and Wardle, 2005). For example, allelopathic compounds of congeneric E. hermaphrodium have strong ecological impacts in boreal forests affecting soil microbiota, tree seedling growth and nutrient uptake among other factors (Nilsson et al., 1993; Nilsson and Wardle, 2005). More specifically in Patagonia, a negative effect of E. rubrum on the growth of other plants has been previously observed in Tierra del Fuego (southern Patagonia), where grasses were not able to recover in sheep-grazed secondary succession terrains that had been colonized by E. rubrum (Collantes et al., 1989). It has been hypothesized that inhibition of the growth of other plants may be mediated by the slow decay of organic matter (Collantes et al., 1989) or a strong allelopathic activity of the species (Mongelli et al., 1997).
Although E. rubrum had a significantly negative impact on the growth of N. pumilio seedlings under both watering regimes, the magnitude of the negative effect was larger under full than under restricted watering. Also, throughout most of the experiment, seedling stomatal conductance (gs) was lower under full-watering conditions than under restricted-watering conditions for seedlings grown with E. rubrum. It is likely that mobilization of water-soluble allelopathic compounds (such as batatasin-III produced by E. hermaphroditum, Odén et al., 1992) would be higher under full-watering conditions, which would explain the stronger inhibitory effect over seedling growth observed in this treatment. A similar decrease in the allelopathic activity of a shrub with increasing environmental stress has been described in semiarid systems (Hortal et al., 2015).
The presence of shrubs can be associated with an increase in soil moisture (Butterfield et al., 2016; Michalet and Pugnaire, 2016). This is consistent with our results which show that this cover type contributed positively to soil volumetric water content (VWC), probably by slowing water loss in comparison to bare soil. This resulted in seedlings not exhibiting water deficit signals under the restricted watering regime because the drop in soil water content at the end of the experiment was most likely insufficient to induce a decrease in seedlings' performance.
In summary, and consistent with the SGH, we found a decrease in the strength of negative interactions in the face of greater abiotic stress. However where allelopathic organisms are involved, this change can be viewed not as an increase in facilitation but as a decrease in conditions that favor interference activity. It must be emphasized that the significantly lower biomass accumulation and relatively high shoot-to-root ratios of seedlings growing with E. rubrum indicate a limitation imposed by E. rubrum on seedling development in spite of the positive effect of the shrub cover on the seedlings gs and water status.
4.2 Potential effects of the biological soil crust on the tree seedlings
In agreement with predictions of the SGH, we found that the effect of BSC on tree seedling growth switched from negative to positive as water availability was reduced. One of the facilitative mechanisms involved could have been improved soil water conservation; a more gradual decrease in soil VWC as water restriction increased in comparison to bare soil. Previous research indicates that the hydrological role of BSC is the result of the interplay among characteristics of BSC, the soil (mainly texture) and the rain event (Whitney et al., 2017). For instance, positive effects on soil moisture have been linked to fine-textured soil, small rain events (Zhang et al., 2008) and well-developed BSC (Whitney et al., 2017), while reductions in soil moisture have been reported for coarse-textured soils and moss-dominated BSC (Xiao et al., 2016) or early successional stages of the BSC (Whitney et al., 2017). In our case, the soil was of intermediate texture and the magnitude of the watering events changed throughout the study; so although relevant these factors may not have played a determining role. The observed effect on soil water more likely was related to the characteristics of the BSC, which was well developed with an important lichen component (Thiet et al., 2014; Whitney et al., 2017).
What is interesting about our findings is how this increase in soil moisture related to seedling performance. When considering the effect of the BSC on the N. pumilio seedlings' gs, we found that it improved as soil water availability was reduced up to a level around 6 % v/v. We presume that the relatively low gs measured at the beginning of the experiment is associated with a negative effect of the activity of the BSC, and that the enhanced seedling physiological performance as drought accentuated could be related to a shift in the balance of the interaction as the BSC desiccated, with the BSC reducing its negative effect on the seedlings while maintaining positive effects on water balance. A competitive role of the BSC is also supported by our results as there was a lack of an effect of soil water on seedling gs, which provides evidence for an active role of the BSC on soil hydrology. Thereafter, severe drought stress gave rise to an abrupt decline in seedling water status, with a soil VWC value around 6 % probably indicating the species threshold for stomatal closure, which is similar to other Nothofagus species (Piper et al., 2007; Varela et al., 2010). Therefore, our results for seedling gs are in agreement with a hump-shaped SGH model.
Another mechanism fostering seedling growth could be related to nitrogen cycling. Previous research indicates that one of the BSC's most important ecological roles is atmospheric carbon and nitrogen fixation (Weber et al., 2016). The BSC used in this study contains the lichen Peltigera polydactylon, which has nitrogen-fixing photobionts. We found that the levels of ammonium and nitrate slightly increased at the end of the experiment in soils covered by the BSC, while they decreased in the other substrate types. This suggests that N may have been added to the system by the BSC during the experimental period.
Our measurements are insufficient to discriminate direct competitive effects of the BSC (Belnap and Lange, 2003; Serpe et al., 2006; Funk et al., 2014; Thiet et al., 2014) from possible indirect effects of BSCs such as interference mediated by allelopathic compounds or unfavorable thermal changes (Soudzilovskaia et al., 2011). Nonetheless, our results do indicate that BSC–plant interactions can change from negative to positive over short time spans and non-linearly over varying water availability. This is in line with previous work indicating that negative and positive effects of BSCs on vascular plants operate together (Thiet et al., 2014), and that the net outcome cannot be linearly predicted by abiotic conditions (Lett et al., 2018). These findings highlight the variability in the response of BSC organisms (Li et al., 2018), and BSC–plant interactions to future climate variations (Weber et al., 2016).
It must be considered that seedling performance was evaluated in this study for only 38 d, and it is necessary to conduct future evaluation over longer periods under field conditions. Nonetheless, results of this study suggest that if Nothofagus pumilio trees recruit in areas presently occupied by a biological soil crust (BSC) or creeping shrub communities such as alpine zones or forest clearings, their subsequent performance under drier conditions could be favored by biotic interactions. In a global context, these results support the premise that biotic interactions and climate change are interdependent. Also, our results highlight the complexity of making predictions about future responses of BSC–plant interactions to climate change. The identity of the organisms comprising the BSC, the characteristics of the underlying soil and the climate of each ecosystem should be acknowledged when making predictions about the effects of climate change on the BSC and subsequent effects on vascular plants. Ultimately our results suggest that some predictions made in the context of global change like upwards movement of altitudinal tree lines need to incorporate the effects of biotic interactions (Hillerislambers et al., 2013).
All original data can be requested from the corresponding author.
CP, IAG, VA and PM conceptualized the work. CP, SAV, VA and PM carried out the assays and acquired data. MGP carried out soil analysis. CP, IG, SAV and OB carried out data analysis. All authors participated in writing and editing.
The authors declare that they have no conflict of interest.
We thank Abel Martinez, Mario Huentu, Maria Marta Azpilicueta, Mario López and Pierre Pitte for their help in producing the seedlings, for data collection, for fieldwork and for producing the map. The manuscript was improved with comments from Agustina Barros, Ricardo Villalba and Mariana Chiuffo. We also thank the National Parks Administration of Argentina. This study was partially funded by ANPCyT (grant: PICT 1417), CONICET (PIP 112-2011010-0809), the Inter-American Institute for Global Change Research (CRN2047) and INTA (PNFOR1104063).
This paper was edited by Daniel Montesinos and reviewed by Rachel Thiet and one anonymous referee.
Armas, C., Ordiales, R., and Pugnaire, F. I.: Measuring plant interactions: a new comparative index, Ecology, 85, 2682–2686, https://doi.org/10.1890/03-0650, 2004.
Barros, V. R., Boninsegna, J. A., Angela, I., Chidiak, M., Magrín, G. O., and Rusticucci, M.: Climate change in Argentina: trends, projections, impacts and adaptation, WIREs Clim. Chang., 6, 151–169, https://doi.org/10.1002/wcc.316, 2014.
Belnap, J.: Biological soil crusts in deserts: a short review of their role in soil fertility, stabilization, and water relations, Arch. Hydrobiol. Suppl. Algol. Stud., 50, 113–126, https://doi.org/10.1127/1864-1318/2003/0109-0113, 2003.
Belnap, J. and Lange, O. L.: Structure and functioning of biological soil crusts: a synthesis, Biol. Soil Crusts Struct. Funct. Manag., 226, 471–479, 2003.
Belnap, J., Weber, B., and Büdel, B.: Biological soil crusts as an organizing principle in drylands, in: Biological soil crusts: an organizing principle in drylands, edited by: Weber, J. B. B. and Budel, B., 3–13, Springer International Publishing, Switzerland, 2016.
Bertness, M. D. and Callaway, R. M.: Positive interactions in communities, Trends Ecol. Evol., 9, 191–193, https://doi.org/10.1016/0169-5347(94)90088-4, 1994.
Bianchi, E., Villalba, R., Viale, M., Couvreux, F., and Marticorena, R.: New precipitation and temperature grids for Northern Patagonia: advances in relation to global climate grids, J. Meteorol. Res., 30, 38–52, https://doi.org/10.1007/s13351-015-5058-y, 2016.
Bowker, M. A., Soliveres, S., and Maestre, F. T.: Competition increases with abiotic stress and regulates the diversity of biological soil crusts, J. Ecol., 98, 551–560, https://doi.org/10.1111/j.1365-2745.2010.01647.x, 2010.
Box, G. E. P. and Jenkins, G. M.: Time series analysis forecasting and control, Revised Ed., Holdem-Day, Inc., San Francisco, USA, 1976.
Breen, K. and Lévesque, E.: Proglacial succession of biological soil crusts and vascular plants: biotic interactions in the High Arctic, Can. J. Bot., 84, 1714–1731, https://doi.org/10.1139/b06-131, 2006.
Brummit, R. K. and Powell, C. E.: Authors of Plants Names, Royal Botanical Garden, Kew, UK, 732 pp., 1992.
Büdel, B.: Biological soil crusts in European temperate and mediterranean regions, in Biological crusts: stracture, function and management, vol. 150, edited by: Belnap, J. and Lange, O. L., 75–86, Springer-Verlag, Berlin Heidelberg, Germany, 2001.
Bustamante-Sánchez, M. A., Armesto, J. J., and Halpern, C. B.: Biotic and abiotic controls on tree colonization in three early successional communities of Chiloé Island, Chile, J. Ecol., 99, 288–299, https://doi.org/10.1111/j.1365-2745.2010.01737.x, 2011.
Butterfield, B. J. and Briggs, J. M.: Regeneration niche differentiates functional strategies of desert woody plant species, Oecologia, 165, 477–87, https://doi.org/10.1007/s00442-010-1741-y, 2011.
Butterfield, B. J., Bradford, J. B., Armas, C., Prieto, I., and Pugnaire, F. I.: Does the stress-gradient hypothesis hold water? Disentangling spatial and temporal variation in plant effects on soil moisture in dryland systems, Funct. Ecol., 30, 10–19, https://doi.org/10.1111/1365-2435.12592, 2016.
Callaway, R. M. and Walker, L. R.: Competition and facilitation: a synthethic approach to interactions in plant communities, Ecology, 78, 1958–1965, 1997.
Calvelo, S. and Liberatore, S.: Catálogo de los líquenes de la Argentina, Kurtziana, 29, 7–170, 2002.
Collantes, M. B., Anchorena, J., and Koremblit, G.: A soil nutrient gradient in Magellanic Empetrum heathlands, Plant Ecol., 80, 183–193, 1989.
del Valle, H. F.: Patagonian soils: a regional synthesis, Ecol. Austral., 8, 103–123, 1998.
Di Rienzo, J. A., Casanove, S., Balzarini, M. G., Gonzalez, L., Tablada, M., and Robledo, C.: InfoStat versión 2017, Universidad Nacional de Córdoba, available at: https://www.infostat.com.ar/?lang=en (last access: 3 April 2019), 2017.
Doxford, S. W., Ooi, M. K. J., and Freckleton, R. P.: Spatial and temporal variability in positive and negative plant – bryophyte interactions along a latitudinal gradient, J. Ecol., 101, 465–474, https://doi.org/10.1111/1365-2745.12036, 2012.
Dullinger, S., Kleinbauer, I., Pauli, H., Gottfried, M., Brooker, R., Nagy, L., Theurillat, J., and Holten, J. I.: Weak and variable relationships between environmental severity and small-scale co-occurrence in alpine plant communities, J. Ecol., 95, 1284–1295, https://doi.org/10.1111/j.1365-2745.2007.01288.x, 2007.
Dvorský, M.: Ecology of alpine plants in NW Himalaya, University of South Bohemia, Czech Republic, 2014.
Etchevehere, P. H.: Los Suelos de la Region Andino-Patagónica, in La Region de los Bosques Andino-Patagonicos, edited by Milan Dimitri, pp. 83–95, Colección Científica de INTA, Buenos Aires, Argentina, 1972.
Ferreyra, M., Ezcurra, C., and Clayton, S.: High Mountain Flowers of the Patagonian Andes, LOLA, Buenos Aires, Argentina, 2006.
Funk, F. A., Loydi, A., and Peter, G.: Effects of biological soil crusts and drought on emergence and survival of a Patagonian perennial grass in the Monte of Argentina, J. Arid Land, 6, 735–741, https://doi.org/10.1007/s40333-014-0022-8, 2014.
Garibotti, I. A., Pissolito, C. I., Villalba, R., Garibotti, I. A., Pissolito, C. I., and Villalba, R.: Spatiotemporal pattern of primary succession in relation to meso-topographic gradients on recently deglaciated terrains in the Patagonian Andes, Arct. Antarct. Alp. Res., 43, 555–567, https://doi.org/10.1657/1938-4246-43.4.555, 2011.
Garreaud, R. D., Lopez, P., Minvielle, M., and Rojas, M.: Large-scale control on the Patagonian climate, J. Climate, 26, 215–231, https://doi.org/10.1175/JCLI-D-12-00001.1, 2013.
Gold, W. G., Glew, C., and Dickson, L. G.: Functional influences of cryptobiotic surface crusts in an alpine tundra basin of the Olympic Mountains, Washington, USA, Northewest Sci., 75, 315–326, 2001.
Gómez-Aparicio, L.: The role of plant interactions in the restoration of degraded ecosystems: A meta-analysis across life-forms and ecosystems, J. Ecol., 97, 1202–1214, https://doi.org/10.1111/j.1365-2745.2009.01573.x, 2009.
He, Q., Bertness, M. D., and Altieri, A.: Global shifts towards positive species interactions increasing environmental stress, Ecol. Lett., 16, 695–706, https://doi.org/10.1111/ele.12080, 2013.
Henríquez, J. M. and Lusk, C. H.: Facilitation of Nothofagus antarctica (Fagaceae) seedlings by the prostrate shrub Empetrum rubrum (Empetraceae) on glacial moraines in Patagonia, Austral Ecol., 30, 877–882, 2005.
Hillerislambers, J., Harsch, M. A., Ettinger, A. K., Ford, K. R., and Theobald, E. J.: How will biotic interactions influence climate change – induced range shifts?, Ann. NY Acad. Sci., 1297, 112–125, https://doi.org/10.1111/nyas.12182, 2013.
Hortal, S., Bastida, F., Moreno, J. L., and Garcia, C.: Benefactor and allelopathic shrub species have different effects on the soil microbial community along an environmental severity gradient, Soil Biol. Biochem., 88, 48–57, https://doi.org/10.1016/j.soilbio.2015.05.009, 2015.
Jacoby, W. G.: Loess: a nonparametric, graphical tool for depicting relationships between variables, Elect. Stud., 19, 577–613, 2000.
Keeney, D. R. and Nelson, D. W.: Nitrogen-inorganic forms, in Methods of Soil Analysis, Part 2, edited by: Miller, R. H. and Keeney, D. R., 643–698, Soil Society of America, Madison, USA, 1982.
Kidron, G. J.: Do mosses serve as sink for rain in the Negev Desert? A theoretical and experimental approach, Catena, 121, 31–39, https://doi.org/10.1016/j.catena.2014.05.001, 2014.
Kitzberger, T., Steinaker, D., and Veblen, T.: Effects of climatic variability on facilitation of tree establishment in northern Patagonia, Ecology, 81, 1914–1924, 2000.
Kuo, S.: Phosphorus, in Methods of Soil Analysis. Part 3. Chemical methods, edited by: Sparks, D. L., Page, A. L., Helmke, P. A., Loeppert, R. H., Soltanpour, P. N., Tabatabai, M. A., and Johnston, C. T., 869–919, Soil Society of America, Madison, USA, 1996.
Langhans, T. M., Storm, C., and Schwabe, A.: Biological soil crusts and their microenvironment: impact on emergence, survival and establishment of seedlings, Flora, 204, 157–168, https://doi.org/10.1016/j.flora.2008.01.001, 2009.
Lenaerts, J. T. M., van den broeke, M. R., van Wessem, J. M., van de Berg, W. J., Van Meijgaard, E., van Ulft, L., and Schaefer, M.: Extreme precipitation and climate gradients in Patagonia revealed by high-resolution regional atmospheric Climate modeling, Am. Meteorol. Soc., 27, 4607–4621, https://doi.org/10.1175/JCLI-D-13-00579.1, 2014.
Lett, S., Wardle, D., Nilsson, M., and Teuber, L. M.: The role of bryophytes for tree seedling responses to winter climate change: Implications for the stress gradient hypothesis, J. Ecol., 106, 1142–1155, https://doi.org/10.1111/1365-2745.12898, 2018.
Li, X., Jia, R., Zhang, Z., Zhang, P., and Hui, R.: Hydrological response of biological soil crusts to global warming: a ten-year simulative study, Glob. Change Biol., 10, 4960–4971, https://doi.org/10.1111/gcb.14378, 2018.
Maestre, F. T., Valladares, F., and Reynolds, J. F.: Is the change of plant – plant interactions with abiotic stress predictable?? A meta-analysis of field results in arid environments, J. Ecol., 93, 748–757, https://doi.org/10.1111/j.1365-2745.2005.01017.x, 2005.
Maestre, F. T., Bowker, M. A., Escolar, C., Puche, M. D., Soliveres, S., Maltez-Mouro, S., García-Palacios, P., Castillo-Monroy, A. P., and Martínez, I.: Do biotic interactions modulate ecosystem functioning along stress gradients? Insights from semi-arid plant and biological soil crust communities, Philos. T. Roy. Soc., 365, 2057–2070, https://doi.org/10.1098/rstb.2010.0016, 2010.
Michalet, R. and Pugnaire, F. I.: Facilitation in communities: underlying mechanisms , community and ecosystem implications, Funct. Ecol., 30, 3–9, https://doi.org/10.1111/1365-2435.12602, 2016.
Mongelli, E., Desmarchelier, C., and Coussi, J.: The potential effects of allelopathic mechanisms on plant species diversity and distribution determined by the wheat rootlet growth inhibition bioassay in South American plants, Rvista Chil. Hist. Nat., 70, 83–89, 1997.
Nilsson, M. and Wardle, D. A.: Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest, Front. Ecol. Environ., 3, 421–428, 2005.
Nilsson, M., Zackrisson, O., and Fengyou, W.: Allelopathic effects by Empetrum hermaphroditum on development and nitrogen uptake by roots and mycorrhizae of Pinus silvestris, Can. J. Bot., 71, 620–628, 1993.
Nuñez, C. I., Raffaele, E., Nuñez, M. A., and Cuassolo, F.: When do nurse plants stop nursing? Temporal changes in water stress levels in Austrocedrus chilensis growing within and outside shrubs, J. Veg. Sci., 20, 1064–1071, 2009.
Odén, P. christopher, Brandtberg, P.-O., Anderson, R., Gref, R., Zackrisson, O., and Nilsson, M.: Isolation and characterization of a germination inhibitor from leaves of Empetrum hermaphroditum Hagerup, Scand. J. For. Reaserch, 7, 497–501, 1992.
Passioura, J. B.: The perils of pot experiments, Funct. Plant Biol., 33, 1075–1079, 2006.
Pendleton, R. L., Pendleton, B. K., Howard, G. L., and Warren, S. D.: Growth and nutrient content of herbaceous seedlings associated with biological soil crusts, Arid L. Res. Manag., 17, 271–281, https://doi.org/10.1080/15324980301598, 2003.
Piper, F. I., Corcuera, L. J., Alberdi, M., and Lusk, C. H.: Differential photosynthetic and survival responses to soil drought in two evergreen Nothofagus species, Ann. For. Sci., 64, 447–452, 2007.
Pugnaire, F. I. and Luque, M. T.: Changes in plant interactions along a gradient of environmental stress, Oikos, 93, 42–49, https://doi.org/10.1034/j.1600-0706.2001.930104.x, 2001.
Raffaele, E. and Veblen, T. T.: Facilitation by nurse shrubs of resprouting behavior in a post-fire shrubland in northern Patagonia, Argentina, J. Veg. Sci., 9, 693–698, https://doi.org/10.2307/3237287, 1998.
Rodríguez-Catón, M., Villalba, R., Morales, M. S., and Srur, A.: Influence of droughts on Nothofagus pumilio forest decline across northern Patagonia, Argentina, Ecosphere, 7, 1–17, https://doi.org/10.1002/ecs2.1390, 2016.
Rolón, A., Mari, C., Fernández, H. M., Dezzotti, A., and Orlov, D.: Efecto del ácido indolbutírico sobre el enraizamiento de estacas juveniles de verano de Nothofagus nervosa y Nothofagus pumilio, Rev. la Fac. Agron. La Plata, 111, 91–98, 2013.
Schmidt, S. K., Reed, S. C., Nemergut, D. R., Grandy, A. S., Cleveland, C. C., Weintraub, M. N., Hill, A. W., Costello, E. K., Meyer, A. F., Neff, J. C., and Martin, A. M.: The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils, P. Roy. Soc. B, 275, 2793–2802, https://doi.org/10.1098/rspb.2008.0808, 2008.
Serpe, M. D., Orm, J., Barkes, T., and Rosentreter, R.: Germination and seed water status of four grasses on moss-dominated biological soil crusts from arid lands, Plant Ecol., 185, 163–178, 2006.
Shi, W., Wang, X., Zhang, Y., Pan, Y., Hu, R., and Jin, Y.: The effect of biological soil crusts on soil moisture dynamics under different rainfall conditions in the Tengger Desert, China, Hydrol. Process., 32, 1–12, https://doi.org/10.1002/hyp.11493, 2018.
Soudzilovskaia, N. A., Graae, B. J., Douma, J. C., Grau, O., Milbau, A., Shevtsova, A., Wolters, L., and Cornelissen, J. H. C.: How do bryophytes govern generative recruitment of vascular plants??, New Phytol., 190, 1019–1031, https://doi.org/10.1111/j.1469-8137.2011.03644.x, 2011.
Srur, A. M., Villalba, R., Rodríguez-Catón, M., and Amoroso, M. M.: Establishment of Nothofagus pumilio at upper treelines across a precipitation gradient in the Northern Patagonian Andes, Arct. Antarct. Alp. Res., 48, 755–766, 2016.
Su, Y.-G., Li, X.-R., Cheng, Y.-W., Tan, H.-J., and Jia, R.-L.: Effects of biological soil crusts on emergence of desert vascular plants in North China, Plant Ecol., 191, 11–19, https://doi.org/10.1007/s11258-006-9210-8, 2007.
Thiet, R. K., Doshas, A., and Smith, S. M.: Effects of biocrusts and lichen-moss mats on plant productivity in a US sand dune ecosystem, Plant Soil, 377, 235–244, https://doi.org/10.1007/s11104-013-2002-8, 2014.
Tylianakis, J., Didham, R., Bascompte, J., and Wardle, D.: Global change and species interactions in terrestrial ecosystems, Ecol. Lett., 11, 1351–1363, https://doi.org/10.1111/j.1461-0248.2008.01250.x, 2008.
Varela, S. A., Gyenge, J. E., Fernández, M. E., and Schlichter, T.: Seedling drought stress susceptibility in two deciduous Nothofagus species of NW Patagonia, Trees, 24, 443–453, https://doi.org/10.1007/s00468-010-0412-2, 2010.
Weber, B., Belnap, J., and Büdel, B.: Synthesis on Biological Soil Crust Research, in: Biological Soil Crusts: An Organizing Principle in Drylands, edited by: Webber, B., Büdel, B., and Belnap, J., 527–534, Springer International Publishing, Switzerland, 2016.
Whitney, K. M., Bradford, J. B., Duniway, M. C., Vivoni, E. R., Belnap, J., and Reed, S. C.: Ecohydrological role of biological soil crusts across a gradient in levels of development, Ecohydrology, 10, 1–18, https://doi.org/10.1002/eco.1875, 2017.
Xiao, B. and Hu, K.: Moss-dominated biocrusts decrease soil moisture and result in the degradation of artificially planted shrubs under semiarid climate, Geoderma, 291, 47–54, https://doi.org/10.1016/j.geoderma.2017.01.009, 2017.
Xiao, B., Hu, K., Ren, T., and Li, B.: Moss-dominated biological soil crusts significantly influence soil moisture and temperature regimes in semiarid ecosystems, Geoderma, 263, 35–46, https://doi.org/10.1016/j.geoderma.2015.09.012, 2016.
Zaady, E., Gutterman, Y., and Boeken, B.: The germination of mucilaginous seeds of Plantago coronopus, Reboudia pinnata, and Carrichtera annua on cyanobacterial soil crust from the Negev Desert, Plant Soil, 190, 247–252, https://doi.org/10.1023/A:1004269031844, 1997.
Zhang, Y. and Belnap, J.: Growth responses of five desert plants as influenced by biological soil crusts from a temperate desert, China, Ecol. Res., 30, 1037–1045, 2015.
Zhang, Y., Aradottir, A. L., Serpe, M., and Bertrand, B.: Interactions of biological soil crusts with vascular plants, in: Biological Soil Crusts: An Organizing Principle in Drylands, edited by: Weber, B., Büdel, B., and Belnap, J., 385–406, Springer International Publishing, Switzerland, 2016.
Zhang, Z.-S., Liu, L.-C., Li, X.-R., Zhang, J.-G., He, M.-Z., and Tan, H.-J.: Evaporation properties of a revegetated area of the Tengger Desert, North China, J. Arid Environ., 72, 964–973, https://doi.org/10.1016/j.jaridenv.2007.11.010, 2008.





